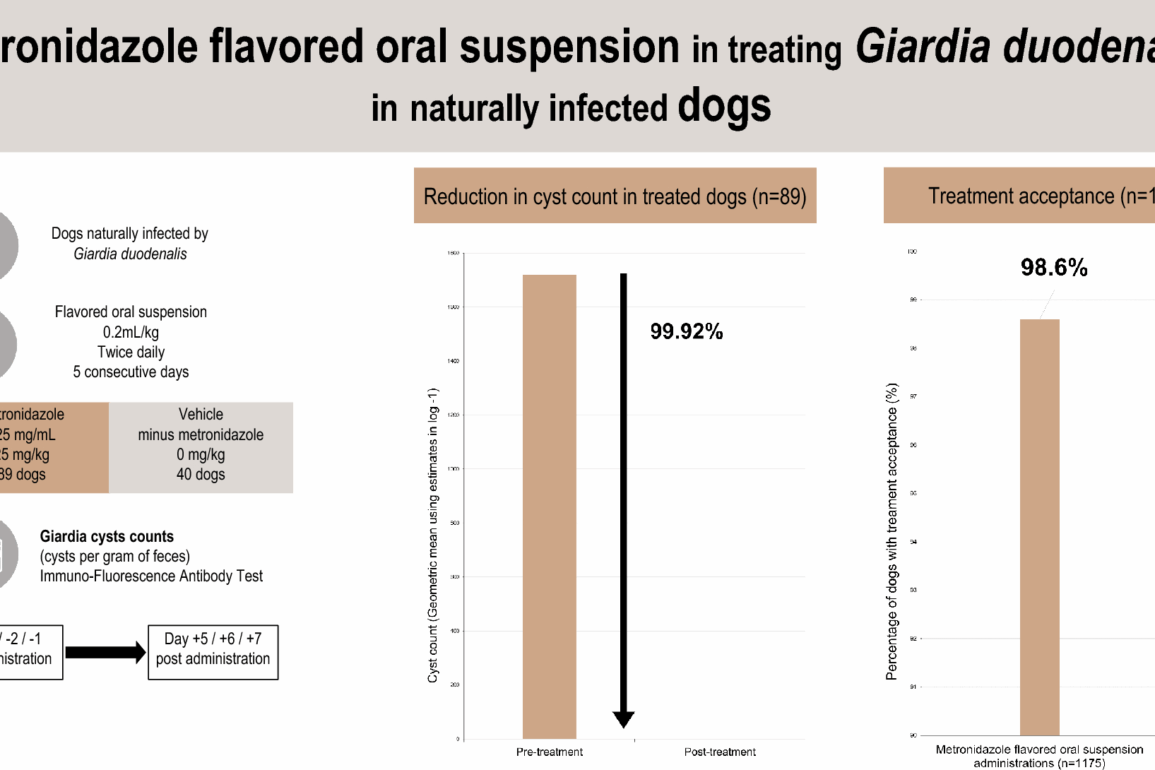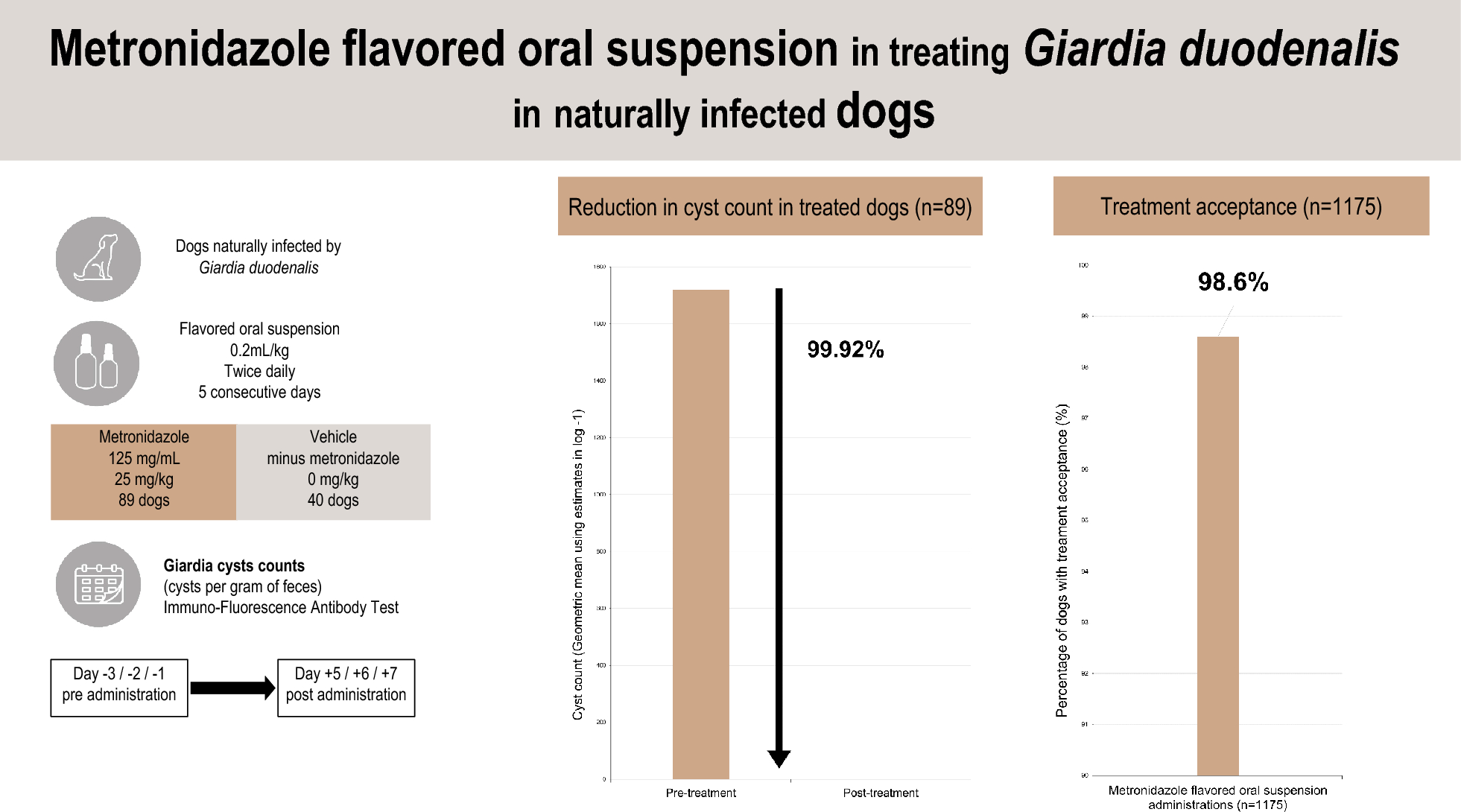
This study demonstrated 99.92% efficacy for AYRADIA administered at 0.2 ml/kg twice daily for 5 days in eliminating G. duodenalis in naturally infected dogs.
The treatment of G. duodenalis infection in dogs, the primary indication for AYRADIA, is strongly supported by the observed marked reduction in cyst shedding. This reduction serves as a direct indicator of successful treatment at the individual level. Furthermore, this substantial decrease in cyst excretion plays a critical role in mitigating environmental contamination, a key determinant in controlling G. duodenalis transmission, particularly within multi-dog populations and households, and has implications for public health considerations [4, 5, 7].
This study integrated metronidazole treatment with hygiene measures, as recommended by global established guidelines [7, 8, 14]. These recommended measures included cleaning the dog with a shampoo, focusing on the peri-anal region, cleaning and disinfecting floors, and cleaning and drying toys, food bowls, clothing, and pet bed. The observed decrease in mean cyst count within the control group could be attributed to the implementation of recommended hygiene measures. The critical importance of hygiene measures must be emphasized as an integral component of successful giardiasis treatment for preventing reinfection in treated dogs and minimizing the risks of relapse and transmission to other animals and human contacts [7, 8, 14].
AYRADIA demonstrated a favorable safety profile, with the incidence of mild and transient gastrointestinal adverse events (diarrhea and emesis) being comparable between the treated and control groups. This suggests that these events were likely associated with individual gastrointestinal sensitivity, rather than a direct consequence of metronidazole administration [8, 14]. This finding indicates that AYRADIA could be administered with a measured risk of adverse reactions in dogs.
Palatability is a crucial factor influencing treatment, both compliance and efficacy. In this trial, AYRADIA demonstrated excellent acceptance (99%). While most dogs readily consume AYRADIA delivered directly into the mouth, the liquid formulation offers the flexibility of administration with food, which can be particularly beneficial for reluctant dogs. The high level of acceptance observed with AYRADIA, despite the inherent taste challenge of this molecule, underscores the high quality standards met in the flavored liquid formulation and the synergistic effects of the ingredients in enhancing palatability [15].
The absence of a direct comparator group could be advanced as a potential limitation in the study design. However, the robust efficacy demonstrated by AYRADIA, coupled with the availability of extensive published literature evaluating and comparing various anti-Giardia agents, provides a comprehensive context for interpreting the findings of this study. A similar study published in 2018 demonstrated 92% efficacy for ERADIA [16]. In this study, metronidazole treatment was compared to fenbendazole treatment [16]. Other molecules have shown efficacy in treating canine giardiasis (ronidazole, oxfenbendazole, nitazoxanide) [7, 17,18,19,20]. A potential limitation in the study design could be the absence of a group receiving treatment without a concurrent hygiene protocol. However, the application of hygiene measures across both groups supports the argument that the observed significant difference in efficacy is primarily attributable to AYRADIA. Furthermore, this approach reflects standard veterinary practice, where hygiene measures are considered an integral component of giardiasis management [7, 10, 11].
The observation of G. duodenalis positivity in dogs without overt clinical signs (diarrhea) underscores the importance of routine screening, particularly in high-risk populations such as puppies and multi-dog households [14, 21]. Rapid tests offer convenient screening in veterinary clinics (e.g., IDEXX SNAP Giardia Test, Virbac Speed Giardia test) [13, 22]. However, positive results require careful interpretation, as they do not confirm active infection or assess treatment success [7]. Understanding each test’s performance metrics (sensitivity, specificity, positive and negative predictive values) is crucial for interpreting results accurately within specific clinical contexts [13, 21]. Direct fluorescent antibody (DFA) testing remains the recommended diagnostic gold standard [14].
The availability of metronidazole formulations specifically designed for canine use is limited, and no other liquid formulations currently exist. The availability of AYRADIA as a liquid oral suspension provides a significant advantage in veterinary practice. This liquid oral suspension facilitates precise, weight-based dosing, particularly crucial for small-breed dogs where tablet splitting can lead to inaccurate administration. Liquid oral suspensions facilitate precise dosing, minimizing the risks associated with inaccurate dosing, such as potential side effects from overdosing or the development of pathogen resistance due to underdosing [14, 19].
Effective management of canine giardiasis is critical for safeguarding both animal and human health. The zoonotic potential of G. duodenalis necessitates prompt and effective treatment to minimize the risk of transmission to human populations [4, 5, 14]. Furthermore, the economic burden associated with prolonged giardiasis, including veterinary costs and potential impact on animal performance, underscores the importance of effective therapeutic interventions [2, 7, 11, 14].
This post was originally published on this site be sure to check out more of their content.