Abstract
Cortisol is widely used in mammals as a measure of HPA axis response. To estimate response to an acute stressor, minimize pain and ease sample collection, salivary cortisol has become preferred over serum cortisol across a variety of species. This includes domestic dogs in which research with laboratory dogs initially demonstrated the predicted relationship between cortisol concentrations in serum and salivary levels sampled within minutes of each other. The Model Population Hypothesis suggests a laboratory dog should be physiologically representative of all dogs. We provide a critical test of this idea by providing the first validation of salivary cortisol against serum measures in healthy puppies less than six months of age (n = 34; 8 to 20-week-old Labrador x Golden Retrievers) as well as a group of healthy adult pet dogs (n = 38). Following previously established methodology, blood and saliva were collected within 4 min of each other. Puppies were sampled multiple times while adults were sampled once. We found that salivary and serum cortisol are poorly correlated in our puppies r(216) = − 0.092, p = 0.178, and adult dogs (r(36) = 0.092, p = 0.582). Our results suggest that previously validated methods with laboratory dogs may not translate to healthy puppies and pet dogs, particularly those less than six months of age. Further research is now needed to identify a salivary sampling method that might allow for this less invasive sampling technique to be used in puppies and pet dogs living in a range of real-world contexts.
Introduction
Physical and psychological stress can have negative consequences on the behavior and health of animals. Nonhuman animals require nonverbal measures of stress and one of the most important and widely used physiological measures is cortisol. Cortisol concentrations are known to reflect activity of the hypothalamic–pituitary–adrenal (HPA) axis and are linked to social behavior, reproductive success, health and psychological well being in a range of animals1,2,3,4.
Dogs have a unique relationship with humans that allow them to perform jobs, but working dogs often perform tasks in high stress environments that include social and non-social stressors5,6,7. Salivary cortisol is the main physiological tool used to capture individual differences in responses to acute stressors in dogs1,2,3,8,9,10. In working dogs, extreme responses have been linked to training outcomes and negative health outcomes11,12,13,14. However, the link between salivary cortisol and dog behavior can be inconsistent. For example, increases in cortisol were correlated with presumed fear behaviors in a pressure wrap study15, but were not associated with behavioral responses in a sound test with working dogs16.
Despite inconsistencies in behavioral studies and blood cortisol concentration being considered the gold standard for measuring HPA response, canine researchers have moved to relying on measuring stress responses using salivary cortisol for several reasons. First, even though cortisol measured in saliva only represents biologically active unbound cortisol, as opposed to total cortisol measured by serum or plasma, previous validation studies found that salivary cortisol samples taken from adult dogs contain roughly 7–12% of cortisol concentrations taken from blood at the same time1,2,3. Second, salivary cortisol has shown to be sensitive to acute changes; in human saliva, increases are detectable in under five minutes17. Third, saliva collection is both significantly less invasive and requires less technical skill than blood collection (e.g.15,16). These characteristics are advantageous when trying to non-invasively assess a dog’s acute physiological response to sudden novel stressors that they will need to endure in a real-life working scenario.
The utility of saliva as a proxy for blood cortisol hinges on a replicable correlation between cortisol concentrations from both measures and the applicability of the validation results from laboratory to non-laboratory populations of dogs. The model population hypothesis (MPH) posits that the specialized selection, rearing, and training experienced by laboratory-housed research animals does not impede the translation of findings to the general population of that same species. The MPH predicts that the laboratory-housed dogs are representative of all dogs—including findings related to behavior, neuroendocrine responses, and HPA axis reactivity. In support of this hypothesis, laboratory dogs have been successfully studied for the development of diagnostic tests, drugs, and vaccines for both veterinary use in dogs and translation to human medicine18,19. Alternatively, the specifics of how dogs are bred and raised can significantly affect trait expression. Physiological measures validated in laboratory dogs might not universally translate to pet dogs. Unlike pet dogs, the genetics, experience, and environments of laboratory dogs are relatively homogenous (i.e. laboratory dogs are typically beagles raised in kennels where factors such as diet, stress, light cycles, and housing conditions are standardized and controlled20).
One challenge to the MPH is the reality that breeding can meaningfully alter neurophysiological systems even after relatively few generations. Fox kits selected for 20–40 generations for friendly behavior towards humans, such as approaching, wagging their tails, and initiating physical contact, showed changes in the adrenal cortex, serotonergic system, and limbic systems associated with a down-regulation of the HPA axis21,22,23. Assistance dogs bred for affiliative behavior have higher levels of free and total oxytocin, but not vasopressin in comparison to pet dogs. Both neuropeptides are associated with affiliative and aggressive behavior24. Differences in size but not breed have been shown to influence cortisol concentrations in dogs, yet no study has investigated if this might affect the correlation of serum and salivary cortisol level25,26.
Another challenge to the MPH is the degree to which environmental factors can shape salivary cortisol results. This includes factors such as swab material, sample size, contamination, housing environment, and sex and neuter/spay status25,26,27,28. And, as highlighted by Chmelíkova et al.29, collection and processing methods for saliva samples are not well standardized, potentially leading to misinterpretations. For example, many studies rely on a comparison of salivary cortisol levels before and after an event (e.g. a stressor), relying on the first sample as a “baseline” of cortisol. However, circadian variation and variability between individuals complicate “baseline” measures1. Whereas these factors may be homogenous in a laboratory dog population, pet dogs who experience more variable and unpredictable environments are likely to include variability in one or more of these factors, potentially reducing the ability for salivary cortisol to accurately measure acute arousal in non-laboratory populations.
While pioneering validation studies established the relationship between serum and salivary cortisol in laboratory dogs, these studies each relied on small groups (n = 6–16) of laboratory raised dogs or on a sample consisting only of diseased dogs that may not be representative of all dogs in all contexts1,2,3,30. A meta-analysis of salivary cortisol studies in dogs noted the significant intra- and inter-individual variability in salivary cortisol and caution about its interpretation at the group level25. The rising popularity in measuring cortisol via saliva sampling nevertheless underscores the need for further confirmation that canine salivary cortisol accurately reflects the activity of the HPA axis in a range of dog populations. If laboratory dogs are a useful model for all dogs, then the correlation between salivary and serum cortisol found in laboratory dogs should be similar in other dog populations.
Here, we test the MPH by examining the prediction that salivary cortisol is a proxy for serum cortisol in healthy adult dogs living in a non-laboratory setting and provide the first validation study in puppies younger than six months of age. We use standard collection procedures, including those recommended by Dreschel and Granger27 that were used in previous validation studies of serum and salivary cortisol in order to replicate the findings of prior work in dogs1,2,3. The MPH is supported if salivary and serum concentrations are correlated in healthy non-laboratory dogs. Alternatively, we will fail to support the MPH if salivary cortisol is not significantly correlated with serum cortisol in these populations. This would suggest that variability in genetics and environment significantly influence cortisol measurements and methodological refinement will be necessary.
Methods
All experimental procedures involving dogs were carried out in accordance with ARRIVE guidelines and were conducted within the approved guidelines of Duke University IACUC protocols A122-21-06 and A128-23-06. We followed the methodology of Dreschel and Granger27 and Vincent and Michell2 as closely as possible in the context of our research setting. These studies are viewed as the seminal validation studies for the use of salivary cortisol in dogs.
Subjects
Puppy sample
100 puppies aged 8–20 weeks were sampled in the study, each having saliva and/or blood collected multiple times across the course of the study (see Tables 1, 2). All puppies were being raised to be assistance dogs through Canine Companions, an organization that breeds and trains dogs for people with disabilities. Puppies were kept with their littermates until 8 weeks of age and then sent to either the homes of volunteer puppy raisers (n = 6) or to Duke University (n = 28) to participate in the Duke/NC State University research program. Puppies who participated in the research program were housed socially during the day with other puppies of the same age and went home with undergraduate students at night. These puppies lived on or near the Duke University campus from 8 to 20 weeks of age and then went to individual puppy raisers. Canine Companions consented to the collection of these data. 218 complete blood-saliva pairs were collected from 34 individuals. A subset of samples (n = 78) was collected before and after an arousing event.
Demographics of the dog participants are shown in Supplementary Table S1. Of the 34 different puppies from which complete serum-saliva paired samples were collected, 21 were males and 13 were females. The mean age of all dogs was 13.39 weeks (range = 8–20 weeks). The number of samples collected from one individual puppy ranged from 1 to 21 (see Supplementary Table S1). The complete paired sample consisted of 29 Labrador × Golden Retriever crosses and 5 Labradors from 24 different litters.
Adult dog sample
A heterogeneous sample (n = 38, M = 22, F = 16) of adult pet dogs from the Raleigh–Durham area were recruited via social media to participate in the study. More than 15 breeds of dogs were represented by the sample (see Supplementary Table S2), but all dogs weighed over 50 pounds to reduce the potential influence of body size on cortisol measurements26. Dogs’ ages ranged from 1 to 15 years (mean = 5.29 years). One sample of blood and saliva was collected from each individual dog. All owners whose dogs participated in the study reviewed and signed an informed consent form.
Procedure
Before collection, all subjects were restricted from eating food or treats for 30 min and restricted from water for at least 10 min (following recommendations by Dreschel and Granger27 to withhold food for at least 20 min). Blood and saliva collection took place between the hours of 9 a.m. and 5 p.m., samples were taken at multiple times of the day, but blood and saliva samples were always taken between 1 and 4 min of each other, in accordance with prior validation work where samples were taken directly one after another without a delay2,3. Puppies were sampled longitudinally. Multiple samples were taken from the same puppy at different weeks of age (see Supplementary Table S1) to evaluate whether the correlation was affected by age. Different numbers of samples were taken from each puppy depending on the ages that owners were able to enter and cease participating in the study. Additionally, if any puppy showed signs of struggle on a given sampling day, a sample was not collected. Some of the puppy samples were taken before a short (1.5 min) arousing event (“pre” samples) and then again 15 min after the event (“post” samples). Puppies rested during the 15-min waiting period in a small room with the same familiar experimenter that set up the arousal event. This event was novel and designed to induce arousal to compare the ability of the two collection methods (blood and saliva) to reflect rises in cortisol concentrations. For the adult dogs, owners brought the dogs into the Duke Canine Cognition Center for an approximately 30-min appointment in which samples were collected using the same collection procedures as was used in the puppy sample. No arousing event was used to manipulate adult cortisol levels as the goal of this sample collection was only to test the association between serum and salivary cortisol in a diverse sample of adult dogs. As with the puppies, any adult dog who struggled or showed signs of distress during sampling was withdrawn and no sample was taken.
Salivary cortisol collection
For collection, a Salimetrics® Children’s Swabs cotton swab was held in the dog’s mouth for 90 s between the lip and gum27, alternating sides after 45 s. The handler sat on the floor next to the dog or with the dog in his or her lap and held the end of the swab opposite from the side that went into the dog’s mouth. An inaccessible small treat or piece of kibble was held in front of the dog’s nose for the duration of the swabbing period to stimulate saliva production; salivary stimulants have not been shown to meaningfully impact salivary cortisol values25. After collection, samples were frozen at − 20 degrees Fahrenheit until shipment to the Salimetrics® laboratory (Carlsbad, CA) on dry ice. At the laboratory, the samples were thawed to room temperature, vortexed, and centrifuged at 3000 RPM for 15 min before assessment using a high-sensitivity enzyme immunoassay with a lower limit of quantification of 0.007 μg/dL (Salimetrics® Salivary Cortisol Immunoassay). Samples were run in duplicate unless low volume prevented a second measurement, and values were averaged. The average intra and inter-assay variance was less than 15%.
Serum cortisol collection
Serum collection was conducted immediately after saliva sample collection to collect the two samples as close together in time as possible (less than 4 min after the saliva collection). For serum collection, dogs were minimally restrained with a food treat for distraction. Between 1.5 and 3 ml of blood was collected fresh with a 23-gauge needle attached to a butterfly catheter and put into an additive-free sterile glass tube. The samples were allowed to clot and within 30 min were placed in a 40-degree Fahrenheit refrigerator until transport. Samples were centrifuged for 12 min at 2500 RPM, and then serum was pipetted off, placed into a plastic clear transfer tube and sent for analysis. The serum collection process took no more than 3 min. All serum samples were collected by a licensed veterinarian, who also monitored dogs’ response to sample collection. Collection was not completed for any dogs who showed signs of stress or resistance to sample collection.
Samples were transported on ice/dry ice to two different laboratories: North Carolina State University (n = 27) and Michigan State University (n = 317). We changed laboratories after discovering that the Michigan State laboratory had improved sensitivity to measure even the smallest cortisol concentrations with accuracy and precision (lower limit of quantification below 1 μg/dL). Blood samples were analyzed using an Immulite 2000 Xpi cortisol immunoassay, which has been validated for canine serum cortisol31. The intra-assay coefficient of variation (CV) was below 10%.
Arousing event
To test cortisol responsiveness and potentially increase cortisol concentrations, a subset of puppy samples was collected immediately before and 15 min after an arousing event. The event was 1 min and 30 s, during which the puppy was placed in a pen with a novel object, an animatronic toy (FurReal toy, Fig. 1) that made unique sounds and movements32. Blood and saliva samples for post-test cortisol were collected 15 min after the test had ended, in accordance with Vincent and Michell’s2 finding that salivary cortisol peaked 11 min after the start of a stressor and remained elevated 30 min after the event, as well as Beerda et al.’s9 results that salivary and serum cortisol increased consistently following a stressor and peaks ~ 15–30 min after the event before normalizing within 60 min. During this 15-min waiting period, the puppy stayed inside the testing pen. A handler was allowed to be in the pen with the puppy but interaction with the puppy was limited during this time (i.e. no cuddling, petting, or speaking in high, encouraging tones). After 15 min, the puppy was taken back to the sample collection area.
Statistical analyses
Welch’s two-sample t-tests were used to compare cortisol concentrations before and after an arousing event for both serum and saliva samples in the puppy sample. Shapiro–Wilk tests were performed to test the normality of distributions for both puppy and adult samples. Spearman’s rho coefficients were then used to assess correlations between salivary and serum cortisol in puppies as an appropriate nonparametric alternative to Pearson correlations, which are influenced by outliers and should not be used on datasets with extreme values33. To ensure that a single individual was not contributing significantly to the model, a Spearman’s rho correlation weighted for the number of samples for each subject was calculated for the mean of serum and salivary cortisol concentrations for each subject. In addition, we conducted a Bland–Altman analysis to evaluate the mean bias between our two measurements of cortisol and calculate the limits of agreement (LoA), which represent the range within which 95% of the differences between the two methods are expected to fall34,35,36. Additionally, a one-sample t-test was performed to determine whether the mean bias was significantly different from zero.
For all tests, statistical significance was set to p < 0.05. Statistical analyses were performed using GraphPad Prism version 10.3.1 for MacOS, (GraphPad Software, Boston, Massachusetts USA, www.graphpad.com), R Statistical Software (R Core Team 2023) and the following R packages: Hmisc, stats.
Results
Serum and salivary cortisol
Puppy sample
218 complete pairs of serum and saliva samples were collected from our puppy sample of 34 individuals (see Table 2 for mean and median serum and salivary concentration values). A Welch’s two-sample t-test was performed to compare cortisol concentrations collected after the arousing event (“post-concentrations”) to those collected before the event (“pre-concentrations”) for both serum and saliva cortisol. Shapiro–Wilk normality tests revealed that both saliva and serum were not normally distributed (both p < 0.001).
Serum cortisol post-concentrations were significantly higher than pre-concentrations, t(157.01) = − 4.104, p = < 0.001. Salivary cortisol post-concentrations, on the other hand, were not significantly different than salivary pre-concentrations, t(156.47) = − 0.487, p = 0.627, indicating that cortisol concentrations did not increase in our saliva samples.
Serum cortisol was not significantly associated with salivary cortisol in this context. A Spearman’s rank correlation was computed to assess the linear relationship between cortisol concentrations in serum and saliva (Fig. 1). There was not a significant correlation between the two variables r(216) = − 0.092, p = 0.178. Further, when grouped by age (weeks), this correlation did not improve with age (see Table 1). In addition, a Spearman’s correlation weighted for the number of samples for each subject did not reveal a strong correlation between serum and salivary concentrations r(33) = − 0.324, p = 0.0614. Thus, in all analyses, puppy serum cortisol concentrations were not found to be positively associated with salivary cortisol concentrations (Fig. 2).
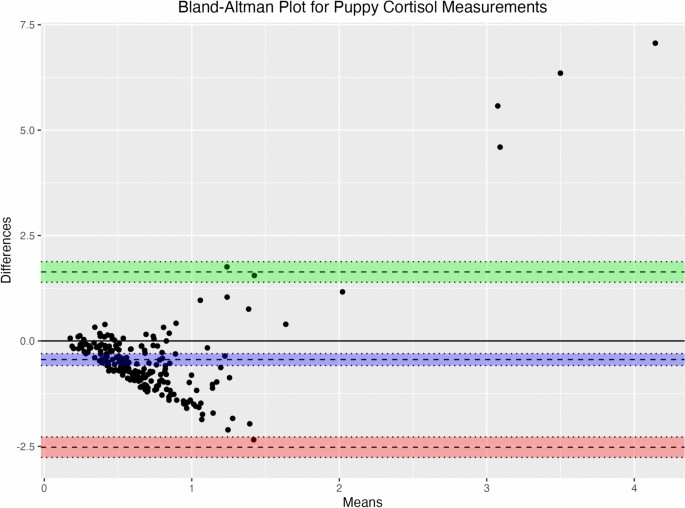
Bland–Altman analysis revealed a statistically significant bias, M = − 0.442, 95% CI [− 0.584, − 0.300], t(217) = − 6.15, p < 0.001, indicating that salivary cortisol values tended to underestimate serum cortisol values. The limits of agreement (LoA) ranged from − 2.521 to 1.637, with corresponding 95% confidence intervals for the upper LoA [1.394, 1.879] and lower LoA [− 2.763, − 2.279]. The spread of data between the lower and upper LoAs was 4.158, and the bias as a proportion of the LoA spread was − 10.632. Further analysis of proportional bias indicated that the bias was − 251.52% of the lowest observed cortisol average and − 10.67% of the highest average. These results suggest a consistent underestimation of serum cortisol by salivary measurements, with a wide range of agreement demonstrating the variability of the magnitude of this underestimation (Fig. 3).
Bland–Altman analysis for puppy cortisol measurements. Bland–Altman plot depicting the variability and proportional bias between serum and salivary cortisol concentrations (μg/dL) in the puppy sample. The blue shaded area indicates the 95% confidence interval around the mean bias (dashed line). The green and red shaded areas showing the 95% confidence intervals around the upper and lower LoA (dashed lines), respectively.
Adult sample
Within the 38 paired samples collected, the mean (median) cortisol concentration was 2.423 (2.052) μg/dL for all the blood samples and 1.489 (0.889) for all the saliva samples. Shapiro–Wilk tests for normality for adult saliva and blood revealed that distributions for both saliva and blood were not normally distributed, indicating a nonparametric test was most appropriate for our dataset. A Spearman’s rank correlation, computed to assess the linear relationship between cortisol concentrations in serum and saliva (Fig. 4), indicated there was no significant correlation between the two variables r(36) = 0.092, p = 0.582.
Bland–Altman analysis revealed a mean bias of − 0.986 μg/dL (95% CI: [− 1.607, − 0.365]), indicating that salivary cortisol measurements consistently underestimated serum cortisol measurements. The limits of agreement (LoA) ranged from − 4.742 μg/dL (95% CI: [− 5.812, − 3.671]) to 2.769 μg/dL (95% CI: [1.698, 3.840]), representing a total spread of 7.51 μg/dL. The large spread between the limits of agreement indicates that the relationship between salivary and serum cortisol is inconsistent, undermining the expectation that salivary cortisol reliably represents 10% of serum cortisol concentrations across the sample. Proportional bias was evident, with the bias representing − 270.57% of the lowest average cortisol value and − 11.92% of the highest average cortisol value. This suggests that salivary cortisol measurements are particularly unreliable at lower cortisol concentrations. A one-sample t-test confirmed that the mean bias was significantly different from zero (t = − 3.215, df = 38, p = 0.0027). Taken together, these results show the consistent underestimation of serum cortisol by salivary cortisol measurements but demonstrates that the magnitude of this underestimation is not consistent. The wide limits of agreement and the presence of proportional bias highlight the substantial variability and poor agreement between the two methods across the full range of cortisol values reported (Fig. 5).
Bland–Altman plot of adult serum and salivary cortisol. Bland–Altman plot depicting the variability and proportional bias between serum and salivary cortisol concentrations (μg/dL) in adult dog sample. The blue shaded area indicates the 95% confidence interval around the mean bias (dashed line). The green and red shaded areas showing the 95% confidence intervals around the upper and lower LoA (dashed lines), respectively.
Discussion
Our study aimed to test the model population hypothesis (MPH) by examining whether salivary cortisol could serve as a reliable proxy for serum cortisol in non-laboratory adult dogs and puppies. The MPH predicts that neuroendocrine characteristics, such as the correlation between serum and salivary cortisol concentrations observed in laboratory-housed dogs, should be valid in more genetically and environmentally variable populations including pet dogs and assistance dog puppies. Beerda et al.3 and subsequent validations found that salivary cortisol concentrations are reliably 7–12% of serum cortisol concentrations in laboratory dogs. In contrast, we did not find that salivary cortisol concentrations were a reliable percentage of serum cortisol concentrations and the measures were not correlated in either assistance dog puppies sampled longitudinally or adult pet dogs.
Our results indicate that the relationship between salivary and serum cortisol is neither consistent nor reliable in non-laboratory dogs. We observed a negative bias of salivary cortisol relative to serum cortisol with wide limits of agreement and significant proportional bias, particularly at lower cortisol concentrations. Proportional bias was particularly evident at lower cortisol concentrations, where the bias reached up to − 270.57%, indicating severe limitations in the ability of salivary cortisol to accurately reflect serum cortisol in low-arousal or baseline states. Interestingly, proportional bias decreased at higher cortisol levels, with bias approaching − 11.92%, closer to the expected proportionality of 10%. This suggests that salivary cortisol may be able to detect higher levels of cortisol, such as after acute stress responses or in conditions associated with elevated HPA activity. Ultimately, however, the substantial variability we report undermines the expectation that salivary cortisol reliably tracks serum cortisol across the full range of serum cortisol values, and that it may not be a reliable measure in non-laboratory dogs.
Our findings that salivary cortisol is particularly unreliable at low cortisol levels has critical implications for its utility in studies employing pre/baseline and post-measure designs. Without accurate baseline measures, detecting meaningful changes following a manipulation becomes challenging, particularly when the manipulation induces small or subtle increases in cortisol. For example, in our study, we observed that an arousal manipulation (introducing a novel toy) successfully increased serum but not salivary cortisol concentrations. The increase in serum cortisol concentrations 15 min after the arousing event demonstrates serum’s (but not saliva’s) ability to reflect an acute stressor via measurement of cortisol. This discrepancy suggests that salivary assays may fail to capture acute HPA axis activation in pet dogs and puppies, limiting salivary cortisol’s usefulness in detecting both absolute levels and relative changes in activation.
Our data suggest that serum cortisol is more reliable than saliva to assess arousal in a non-laboratory population of dogs. Our initial prediction that salivary cortisol would serve as a reliable proxy for serum cortisol in both puppy and adult populations was not supported by our findings. We found that cortisol concentrations in serum and salivary samples were not well-correlated in 34 puppies younger than 6 months of age or in a heterogenous healthy adult sample of 38 adult pet dogs, despite previous work showing a strong correlation between serum and salivary cortisol concentrations from laboratory dogs1,2,3. We also observed that an arousal manipulation (introducing a novel toy) successfully increased serum but not salivary cortisol concentrations. This indicates that the hypothalamic–pituitary–adrenal (HPA) axis was in fact activated by this manipulation, and that this arousal was reflected in serum cortisol concentrations. However, the manipulation did not consistently result in a corresponding increase in salivary cortisol concentrations. This indicates that salivary assays may not capture the acute activation of the HPA axis in pet dogs and puppies, as they do in laboratory dogs.
The limitations of salivary cortisol as a proxy for serum cortisol become apparent when comparing statistical approaches with prior validation studies. Beerda et al.3 accounted for outliers by removing values greater than three times the interquartile range but did not report removing low outliers or testing for normality before performing a parametric Pearson correlation. Since Pearson correlations are easily influenced by extreme values, uncertainty about the normality of Beerda et al.’s3 dataset raises questions about the appropriateness of these authors’ analyses for this dataset and if this could have resulted in misleading findings33. Vincent and Michell2 also performed parametric Pearson correlations without testing for normality and did not report the removal of any outliers. Our null findings in our Spearman’s rho analyses of the correlation between our serum and saliva samples suggest that existing validations of salivary cortisol assays may not translate to puppies and pet dogs living outside of a laboratory.
The discrepancy between serum and salivary cortisol concentrations seen in our data may arise from environmental and genetic factors that were not present in earlier validations. Although variations in salivary cortisol concentrations across breeds have been documented37, these differences should not inherently disrupt the correlation with serum cortisol. However, non-laboratory environments introduce additional genetic and environmental variables that could significantly impact salivary cortisol measurements, challenging the general applicability of salivary cortisol as a reliable proxy across all dog populations. It has been well-documented that environmental and collection factors can influence cortisol concentrations in salivary samples, including volume, swab material, housing style, and environmental contamination27,38. In addition, increased metabolic demand in response to stress is reflected in elevated glucocorticoid secretion such as cortisol4. This might contribute to the differences observed between laboratory and non-laboratory dogs since in laboratory settings, dogs typically experience consistent and controlled environments, such as regulated diets, altered light cycles, standardized exercise routines, and minimal exposure to sudden stressors20. The prior validations of serum-salivary symmetry in healthy dogs from Beerda et al.3, Vincent and Michell2 and Giannetto et al.1 all collected samples from a small number of laboratory dogs (n = 16, n = 6, n = 6, respectively). Of these validations, only Giannetto et al.1 confirmed a normal distribution of their dataset before performing parametric analysis. In contrast, our datasets for all samples were not normally distributed, which could be an indicator of intra- and/or inter-individual variability in salivary cortisol measurements as has been suggested by Cobb et al.25. Controlled laboratory environments likely result in a more stable metabolic rate in dogs, potentially leading to a more predictable relationship between serum and salivary cortisol. In contrast, pet dogs, who live in more variable environments, are subject to a wider range of stressors, both psychological and physiological. Cortisol concentrations in pet dogs may not solely indicate psychological stress but also reflect an underlying metabolic response to environmental challenges. While reactivity measures (pre- and post-arousal manipulation) can help account for some of these variables, they may not completely isolate psychological stress from physiological stress. Kang et al.30 reported a significant positive correlation between serum and salivary cortisol in 35 client-owned diseased dogs, but did not report sample characteristics that may have influenced results such breed, size, or age. These authors also reported that while dogs’ pain scores and serum cortisol concentrations decreased after surgical intervention for pain, salivary cortisol concentrations did not, suggesting a discrepancy between the two measures30. By recognizing that cortisol can reflect a broader range of stressors, we underscore the importance of considering both metabolic and psychological factors when using cortisol as a measure of stress in healthy, non-laboratory dog populations.
Given the robustness of our design and the observed effects of our manipulation, it is unlikely that methodological factors alone account for the poor correlation. Although low sample volume in small dogs can artificially reduce measurement values, our puppy samples had adequate volume for sample processing28. There could be a delay in the peak concentration of cortisol in saliva versus serum that impacted our investigation, in dairy cattle, salivary cortisol was shown to peak 10–15 min after a peak in serum cortisol following a stressful event. A delay in dogs has been suggested by Vincent and Mitchell2 but not shown to negate the correlation between serum and saliva, and all previous validation studies conducted in dogs collected serum and saliva without a delay time1,2,3,39. Beerda et al.3 collected simultaneous blood and saliva samples from dogs repeatedly at increments ranging from 5 to 30 min yet report no delay in salivary response relative to plasma. Other work assessing the time course of salivary cortisol increase after an acute stressor in dogs has shown that concentrations were significantly elevated 10, 15, 20, and 30 min after a stressor, and normalized within 60 min of the event9; a similar time course has also been shown in humans17. A delay between salivary and serum cortisol would not explain why, in our puppy sample, salivary cortisol would not have increased in samples taken 15 min after an arousing event. We also followed the standard practice of withholding food 30 min prior to the first salivary collection Dreschel and Granger27 recommend withholding for at least 20 min). It is of note that no study has investigated the time course at which food interferes with salivary cortisol concentrations in dogs, but a meta-analysis of salivary cortisol in canine studies reports no significant effects of eating within 3 h of sampling25. In humans, a protein rich diet has been shown to increase salivary cortisol concentrations for up to 2 h40. While we did not randomize the order of collection (we always collected saliva prior to blood), this order minimized restraint and potential resulting increases in cortisol concentrations in blood38. Our blood collections were performed using a food distraction which would preclude immediate collection of saliva. While it is possible that the short delay between serum and salivary sample collection contributed to these results, this delay was 1–2 min for most samples and no more than 4 min for any sample. In humans, a delay of 1–2 min for unbound cortisol to pass through cell membranes into saliva has been reported, which aligns with our collection timing41. Since a delay has not been shown meaningfully in dogs, we would still expect serum and salivary concentrations to be associated as has been shown in laboratory adult dogs9. Since prior work has demonstrated that handling effects are not seen until after at least 4 min, it is unlikely that restraint from blood or saliva collection impacted our results when both of our samples were collected in less than 4 min38. Further, if handing from salivary collection did increase subsequent serum cortisol concentrations, our results might reflect high salivary concentrations relative to the expected 7–12% of serum concentrations. This effect was not seen in our sample, where we report salivary concentrations as low as − 270% of serum values taken at the same timepoint. We also report qualitatively that that blood collection did not lead to significant struggling or resistance by puppies, as assessed by a veterinary behaviorist (MEG). While it remains possible that specific methodological choices contributed to our null result, we followed all previous recommendations for serum and saliva collection and processing25,27,38.
The results raise questions about the generalizability and reliability of salivary cortisol as a suitable proxy for serum in pet and assistance dogs and emphasizes the need for further research to understand the dynamics of cortisol measurement in dogs. To our knowledge, the correlation between unbound cortisol circulating in saliva and bound cortisol in blood has only been explicitly investigated in adult laboratory dogs. Our data fails to validate the use of saliva samples as a replacement for blood when measuring cortisol levels in pet adult dogs and puppies younger than 6 months of age. The poor correlation we observed suggests that factors beyond methodology may contribute to the discrepancy between serum and salivary cortisol concentrations, or that current methodological recommendations fail to account for unknown confounding factors. Our study emphasizes the need for further research to understand the dynamics of cortisol measurement in diverse dog populations. The development of new methods to measure acute stress in dogs, such as salivary alpha amylase, which has shown promise in assessing chronic stress associated with disease state, will be required to accurately measure cortisol in these populations42. Until such methods are validated, we recommend serum collection over salivary collection to measure acute changes in cortisol concentrations in pet dogs and puppies. Although collection of serum cortisol increases the burden of collection and the potential for pain, making salivary cortisol an attractive option as a biomarker for stress, we suggest that serum may be necessary to accurately assess acute HPA activation in dogs of this age. Future work should investigate why serum and salivary cortisol concentrations are not associated in healthy, pet dogs and whether this discrepancy is due to the limitations of current salivary assays or other underlying factors.
Data availability
The datasets generated by this research and analyzed during the current study are available in the DRYAD repository, http://datadryad.org/stash/share/WvWLxluWOrJh_M1jk5tvFjmDP8q8CUugT7JbC4b-ixg.
References
-
Giannetto, C. et al. Parallelism of circadian rhythmicity of salivary and serum cortisol concentration in normal dogs. J. Appl. Biomed. 12(4), 229–233. https://doi.org/10.1016/j.jab.2014.01.009 (2014).
-
Vincent, I. C. & Michell, A. R. Comparison of cortisol concentrations in saliva and plasma of dogs. Res. Vet. Sci. 53(3), 342–345. https://doi.org/10.1016/0034-5288(92)90137-Q (1992).
-
Beerda, B., Schilder, M. B., Janssen, N. S. & Mol, J. A. The use of saliva cortisol, urinary cortisol, and catecholamine measurements for a noninvasive assessment of stress responses in dogs. Horm. Behav. 30(3), 272–279. https://doi.org/10.1006/hbeh.1996.0033 (1996).
-
Emery Thompson, M. Energetics of feeding, social behavior, and life history in non-human primates. Horm. Behav. 91, 84–96. https://doi.org/10.1016/j.yhbeh.2016.08.009 (2017).
-
Brady, K., Cracknell, N., Zulch, H. & Mills, D. S. A systematic review of the reliability and validity of behavioural tests used to assess behavioural characteristics important in working dogs. Front. Vet. Sci. https://doi.org/10.3389/fvets.2018.00103 (2018).
-
Lazarowski, L. et al. Selecting dogs for explosives detection: Behavioral characteristics. Front. Vet. Sci. https://doi.org/10.3389/fvets.2020.00597 (2020).
-
Rooney, N. J., Clark, C. C. A. & Casey, R. A. Minimizing fear and anxiety in working dogs: A review. J. Vet. Behav. 16, 53–64. https://doi.org/10.1016/j.jveb.2016.11.001 (2016).
-
Beerda, B. et al. Chronic stress in dogs subjected to social and spatial restriction. II. Hormonal and immunological responses. Physiol. Behav. 66(2), 243–254 (1999).
-
Beerda, B., Schilder, M. B., Van Hooff, J. A., De Vries, H. W. & Mol, J. A. Behavioural, saliva cortisol and heart rate responses to different types of stimuli in dogs. Appl. Anim. Behav. Sci. 58(3–4), 365–381 (1998).
-
Wenger‐Riggenbach, B. et al. Salivary cortisol concentrations in healthy dogs and dogs with hypercortisolism. J. Vet. Int. Med. 24(3), 551–556. https://doi.org/10.1111/j.1939-1676.2010.0494.x (2010).
-
Dollion, N. et al. Fear/Reactivity in working dogs: An analysis of 37 years of behavioural data from the Mira Foundation’s future service dogs. Appl. Anim. Behav. Sci. 221, 104864 (2019).
-
Haverbeke, A., De Smet, A., Depiereux, E., Giffroy, J.-M. & Diederich, C. Assessing undesired aggression in military working dogs. Appl. Anim. Behav. Sci. 117(1), 55–62. https://doi.org/10.1016/j.applanim.2008.12.002 (2009).
-
Haverbeke, A., Diederich, C., Depiereux, E. & Giffroy, J. M. Cortisol and behavioral responses of working dogs to environmental challenges. Physiol. Behav. 93(1), 59–67. https://doi.org/10.1016/j.physbeh.2007.07.014 (2008).
-
van Houtert, E. A. E., Endenburg, N., Rodenburg, T. B. & Vermetten, E. Do service dogs for veterans with PTSD mount a cortisol response in response to training?. Animals 11(3), Article 3. https://doi.org/10.3390/ani11030650 (2021).
-
Pekkin, A.-M. et al. The effect of a pressure vest on the behaviour, salivary cortisol and urine oxytocin of noise phobic dogs in a controlled test. Appl. Anim. Behav. Sci. 185, 86–94. https://doi.org/10.1016/j.applanim.2016.09.003 (2016).
-
Gruen, M. E. et al. The use of an open-field model to assess sound-induced fear and anxiety-associated behaviors in Labrador retrievers. J. Vet. Behav. 10(4), 338–345. https://doi.org/10.1016/j.jveb.2015.03.007 (2015).
-
Becker, L. & Rohleder, N. Time course of the physiological stress response to an acute stressor and its associations with the primacy and recency effect of the serial position curve. PLoS ONE 14(5), e0213883. https://doi.org/10.1371/journal.pone.0213883 (2019).
-
Rossi, G. et al. A conventional beagle dog model for acute and chronic infection with Helicobacter pylori. Infect. Immun. 67(6), 3112–3120 (1999).
-
Schulte, E. & Arlt, S. P. What kinds of dogs are used in clinical and experimental research?. Anim. Open Access J. MDPI 12(12), 1487. https://doi.org/10.3390/ani12121487 (2022).
-
Bolman, B. Dogs for life: Beagles, drugs, and capital in the twentieth century. J. Hist. Biol. 55(1), 147–179 (2022).
-
Kulikov, A. V., Zhanaeva, E. I. & Popova, N. K. Change in tryptophan hydroxylase activity in the brain of silver foxes and wild Norway rats in the course of selection according to behavior. Genetika 25(2), 346–350 (1989).
-
Popova, N. K. From genes to aggressive behavior: The role of serotonergic system. BioEssays 28(5), 495–503 (2006).
-
Trut, L. N. Early canid domestication: The Farm-Fox Experiment: Foxes bred for tamability in a 40-year experiment exhibit remarkable transformations that suggest an interplay between behavioral genetics and development. Am. Sci. 87(2), 160–169 (1999).
-
MacLean, E. L. et al. Endogenous oxytocin, vasopressin, and aggression in domestic dogs. Front. Psychol. https://doi.org/10.3389/fpsyg.2017.01613 (2017).
-
Cobb, M. L., Iskandarani, K., Chinchilli, V. M. & Dreschel, N. A. A systematic review and meta-analysis of salivary cortisol measurement in domestic canines. Domest. Anim. Endocrinol. 57, 31–42. https://doi.org/10.1016/j.domaniend.2016.04.003 (2016).
-
Sandri, M., Colussi, A., Perrotta, M. G. & Stefanon, B. Salivary cortisol concentration in healthy dogs is affected by size, sex, and housing context. J. Vet. Behav. 10(4), 302–306 (2015).
-
Dreschel, N. A. & Granger, D. A. Methods of collection for salivary cortisol measurement in dogs. Horm. Behav. 55(1), 163–168 (2009).
-
Harmon, A. G., Hibel, L. C., Rumyantseva, O. & Granger, D. A. Measuring salivary cortisol in studies of child development: Watch out—What goes in may not come out of saliva collection devices. Develop. Psychobiol. J. Int. Soc. Develop. Psychobiol. 49(5), 495–500 (2007).
-
Chmelíková, E. et al. Salivary cortisol as a marker of acute stress in dogs: A review. Domest. Anim. Endocrinol. 72, 106428 (2020).
-
Kang, E.-H., Park, S.-H., Oh, Y.-I. & Seo, K.-W. Assessment of salivary alpha-amylase and cortisol as a pain related stress biomarker in dogs pre-and post-operation. BMC Vet. Res. 18(1), 31. https://doi.org/10.1186/s12917-021-03114-2 (2022).
-
Korchia, J. & Freeman, K. P. Validation study of canine serum cortisol measurement with the Immulite 2000 Xpi cortisol immunoassay. J. Vet. Diagn. Invest. 33(5), 844–863 (2021).
-
Gnanadesikan, G. E. et al. Dog cognitive development battery methods. OSF. https://doi.org/10.17605/OSF.IO/CSK3A (2023).
-
De Winter, J. C., Gosling, S. D. & Potter, J. Comparing the Pearson and Spearman correlation coefficients across distributions and sample sizes: A tutorial using simulations and empirical data. Psychol. Methods 21(3), 273 (2016).
-
Dorn, L. D., Lucke, J. F., Loucks, T. L. & Berga, S. L. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 44(Pt 3), 281–284. https://doi.org/10.1258/000456307780480954 (2007).
-
Giavarina, D. Understanding Bland Altman analysis. Biochemia Medica 25(2), 141–151 (2015).
-
Martin Bland, J. & Altman, DouglasG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 327(8476), 307–310. https://doi.org/10.1016/S0140-6736(86)90837-8 (1986).
-
Pasha, S. et al. The saliva proteome of dogs: Variations within and between breeds and between species. PROTEOMICS, 18(3–4), 1700293. https://doi.org/10.1002/pmic.201700293 (2018).
-
Kobelt, A., Hemsworth, P., Barnett, J. & Butler, K. Sources of sampling variation in saliva cortisol in dogs. Res. Vet. Sci. 75(2), 157–161 (2003).
-
Hernandez, C. E. et al. Time lag between peak concentrations of plasma and salivary cortisol following a stressful procedure in dairy cattle. Acta Vet. Scand. 56(1), 61. https://doi.org/10.1186/s13028-014-0061-3 (2014).
-
Gibson, E. L. et al. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom. Med. 61(2), 214–224. https://doi.org/10.1097/00006842-199903000-00014 (1999).
-
Kirschbaum, C. & Hellhammer, D. H. Salivary cortisol in psychobiological research: An overview. Neuropsychobiology 22(3), 150–169 (1989).
-
Hong, H.-R., Oh, Y.-I., Kim, Y. J. & Seo, K.-W. Salivary alpha-amylase as a stress biomarker in diseased dogs. J. Vet. Sci. 20(5), e46. https://doi.org/10.4142/jvs.2019.20.e46 (2019).
Acknowledgements
Thank you to the incredible staff, volunteers, and puppy raisers at Canine Companions, Guiding Eyes for the Blind, and Ears, Eyes, Nose and Paws for their dedication and hard work coordinating this project. Thank you to the past and present Duke Canine Cognition Center team for coordinating puppy care and testing for this project, especially Candler Cusato, Gabrielle Bunnell, and Emma Nelson. Thank you to all the research assistants who participated in dog handling for this project.
Funding
This work was funded in part by grants from the Office of Naval Research (N00014-16-12682), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH-1RO1HD097732) and the American Kennel Club Canine Health Foundation (Grant-#02700).
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, investigation, all authors; Writing and figures—Original Draft, M.F.; Writing—Reviewing and editing, all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All experimental procedures involving dogs were carried out in accordance with ARRIVE guidelines. This work was conducted under Duke University IACUC protocols A122-21-06 and A128-23-06, and all procedures were conducted within these approved guidelines.
Human and animal rights and informed consent
Participation in all behavioral testing was voluntary. Behavior during blood and saliva collection was monitored a veterinary behaviorist (MEG) such that subjects who displayed signs of stress could opt out of collection. There were no human subjects in this research and thus informed consent is not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ferrans, M., Salomons, H., Moore, K. et al. Salivary cortisol is an unreliable correlate of serum cortisol in adult pet dogs and assistance dog puppies.
Sci Rep 15, 15986 (2025). https://doi.org/10.1038/s41598-025-00425-4
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-00425-4
Keywords
This post was originally published on this site be sure to check out more of their content.