Abstract
Impaired social interaction and repetitive behavior are key features observed in individuals with autism spectrum disorder (ASD). SHANK3 is a high-confidence ASD risk gene that encodes an abundant scaffolding protein in the postsynaptic density. In wild-type (WT) domestic dogs, maternal behaviors such as licking and nursing (largely milk feeding) of puppies are most commonly observed. To address whether SHANK3 plays a role in social behaviors especially maternal behaviors, we analyzed Shank3 mutant dogs generated by CRISPR/Cas9 methodology. We found that Shank3 mutant dams exhibited a fewer and shorter licking behavior, as well as reduced nursing frequency when compared with WT dams. Additionally, a significant decrease in blood oxytocin (OXT) concentration was detected in Shank3 mutant dams. We thus conducted a vehicle-controlled experiment to examine whether a two-week intranasal OXT treatment, initiated on the 8th postpartum day, could rescue the maternal licking deficits in Shank3 mutant dams. We found that the decreased licking behavior in Shank3 mutant dams was significantly attenuated both acutely and chronically by OXT treatment. The rescue effect of OXT implicates an oxytocinergic contribution to the maternal defects in Shank3 mutant dams, suggesting a potential therapeutic strategy for SHANK3-associated ASD.
Highlights
-
Maternal licking behavior is impaired in an autism-associated dog model carrying Shank3 mutations.
-
Serum oxytocin levels are reduced in Shank3 mutant dams.
-
Both acute and chronic intranasal oxytocin treatment rescues maternal licking behavior defects in Shank3 mutant dams.
Introduction
Animals perform many activities throughout their lives for survival and reproduction. They search for food and mates, defend themselves, and often care for their offspring or others. This series of interactions among individuals of the same species is collectively referred to as social behavior [1]. Parental behavior is a distinct naturalistic social behavior, including feeding and protection of young, that is essential for survival as well as the mental and physical well-being of the offspring in many species [2]. In mammals, mothers commonly take the primary responsibility of providing food, warmth, and protection for their offspring [3]. The maternal behavior of domestic dogs mainly consists of nursing (i.e., milk feeding) and licking [4, 5].
Autism spectrum disorder (ASD) is a set of heterogeneous neurodevelopmental disorders, characterized by impaired social interaction and repetitive behavior [6, 7]. Mutations in SH3 and multiple ankyrin repeat domains protein 3 (SHANK3), an abundant postsynaptic scaffolding proteins at excitatory synapses, are the first and one of the most replicated genetic risks for idiopathic ASD [8,9,10,11,12]. We recently generated Shank3 mutant dogs which showed distinct and pronounced social behavior deficits, including social withdrawal and reduced interactions with humans in different settings [8].
Previous studies have examined some aspects of motherhood in ASD mothers, including pregnancy, childbirth, and baby caring, through questionnaire surveys, where the ASD mothers reported difficulties in processing sensory experiences, such as breastfeeding issues, as well as challenges in communicating with professionals, such as clinicians, midwives, and nurses [13,14,15]. However, there is a lack of objective description of maternal behaviors of ASD mothers after giving birth to babies.
Maternal behavior toward infants is sensitive to physiological and environmental factors such as stress and hormone levels. Hormones such as oxytocin (OXT), estrogen, and prolactin regulate maternal behavior [16, 17]. OXT is mainly synthesized in the supraoptic nucleus and paraventricular nucleus (PVN) of the hypothalamus, and released to the peripheral blood and multiple regions in the central brain [18]. In a recent study, two mutually exclusive projection patterns of OXT neurons in the PVN was revealed by single-neuron projectomes [19]. Cluster 1 neurons have relatively short axons that terminate exclusively in the median eminence for peripheral release, while cluster 2 neurons send wide-spread axons to over two hundred brain areas beyond the median eminence [19].
Converging evidence implicates OXT in maternal behaviors. Intracerebroventricular (ICV) administration of OXT to virgin female rats induced a rapid onset of full maternal behavior [20]. In non-pregnant female sheep, ICV infusions of OXT can evoke both maternal responses and bonding with lambs [21]. In addition, one study showed that visually observing maternal retrieval activates OXT neurons and promotes alloparenting in virgin mice [22], while another study found that OXT neurons respond to pup vocalizations, but not to pure tones, through input from the posterior intralaminar thalamus [23]. Mutations in OXT or OXT receptors in rodent models have been found to exhibit impaired maternal behavior [24, 25], as well as multiple social behavioral deficits [26, 27], and repetitive behaviors [28]. These studies demonstrate that damage to the OXT system in rodents leads to a range of ASD-related symptoms.
There is no effective medication for the core social symptoms of ASD. OXT has been reported to be a powerful regulator of social behavior by enhancing social reward, empathy and promoting the sensory detection and evaluation of social cues [29,30,31]. Several studies have established a central effect of intranasal OXT in ASD patients based on functional magnetic resonance imaging studies [32, 33]. Over the past two decades, multiple clinical trials have investigated the effects of OXT intervention in the clinical manifestations of patients with ASD [33,34,35,36]. However, their findings are ambivalent. It is noteworthy that a randomized controlled trial of intranasal OXT does not appear statistically efficacious in improving the social behavior in 18 Phelan-McDermid syndrome patients carrying SHANK3 mutations aged 5–17 [34]. The inconsistent results among these various investigations may have been the result of differences in cohort age, sample size, OXT formulation or dose, treatment duration, social context, baseline levels of OXT, outcome measures, or analytical methods.
In the present study, we found that maternal licking behavior is impaired in Shank3 mutant dams. To determine the hormones responsible for the regulation of maternal behaviors, we examined the blood levels of multiple hormones and found that OXT was specifically decreased in Shank3 mutant dams. We further revealed significant acute and chronic beneficial effects of OXT treatment on maternal behavior in Shank3 mutant dams. Our findings indicated an unexpected role for Shank3 in the regulation of maternal behavior through OXT and suggest a promising role of OXT in ameliorating the social behavior deficits in, at least, a subset of ASD patients.
Materials and methods
Subjects
All of the dogs analyzed in this study were between 1 and 3 years old. The animals were generally housed in 1.0 × 1.0 × 1.0 m (length x width x height) home-cage individually and transferred to the whelping room approximately one week before the estimated delivery day. They were individually housed in 1.2 × 1.2 × 1.2 m (length x width x height) whelping cage. The humidity of the whelping room was 40–60% and the temperature was 22–24 °C. There were windows that admitted natural light in the whelping room. For gestation and lactation dogs, a special canine chow which is rich in protein, fat, as well as vitamins and minerals with more nutrition and higher energy (Royal Canin Pet Food, Shanghai, China) was provided twice daily during 8:00–11:00 and 16:00–18:00. Dogs not in production period were fed maintenance food (Beijing keao Xieli Feed, Beijing, China) twice daily. Water was available from an automatic watering device. Dams stayed with their puppies until they were weaned at the age of eight weeks.
Whelping was monitored via a camera (Hikvision, Hangzhou, China) at the ceiling. In the whelping cage, there was a 0.9 × 0.6 × 0.15 m (length x width x height) whelping box. The flooring in the box was a soft bed. Puppies were weighed every day at the first three weeks and were vaccinated in accordance with veterinary’s recommendations. The puppies were vaccinated canine distemper-parvovirus vaccine (modified live virus) Nobivac® Puppy DP (Merck Animal Health, New Jersey, USA) at 21 days old. They were vaccinated Canine Distemper-Adenovirus Type 2-Coronavirus-Parainfluenza-Parvovirus Vaccine (Modified Live and Killed Virus) Leptospira Canicola-Icterohaemorrhagiae Bacterin Vanguard® Plus 5-CVL (Zoetis, Lincoln, Nebraska, USA) at 42, 63 and 84 days old. The puppies were vaccinated canine and feline rabies vaccine (Inactivated) Nobivac® Rabies (Merck Animal Health, New Jersey, USA) at 93 days old. Beginning in the fifth week, puppies were allowed access to solid puppy food which is easy to digest, and rich in protein, vitamins, and minerals. (Royal Canin Pet Food, Shanghai, China).
All behavioral tests were performed at similar time of the day (9:00–12:00). All the mutant dams and WT controls listed in Table 1. The life experience of each animal was kept as similar as possible to minimize individual variability.
Video recording and analysis of dam-puppy interaction
Parturitional responses, such as licking the amniotic membrane enveloped puppies, biting and consuming the umbilical cord and placenta immediately after parturition (PP0) [16], were recorded with a surveillance camera, and all video files were stored for later analysis.
For analysis of puppy directed maternal behaviors, the following parameters were scored on PP1: licking, nursing, and the time spent in the whelping box. The duration and times of licking and nursing, and the time in the whelping box were scored in a 60-min video between 6:00–9:00 am, starting when the dam returned to the whelping box after her first morning urination and/or defaecation.
Licking behavior was defined as any movement of the dam’ tongue along the genitalia, anus, belly and rear of a puppy [37, 38]. Nursing behavior was defined as at least one puppy lined up and had its mouth around the udder with or without milk feeding [38, 39]. Dam in whelping box was defined as the dam had at least one paw within the whelping box [38].
Recorded videos of 60 min between 6:00–9:00 am were analyzed manually by three trained scorers who were blind to the genotype. Inter-scorer reliability was determined by providing each scorer with 3 × 60-min of video samples (all scorers analyzed the same videos). The average inter-scorer reliability between the scorers was 90%, ranging from 100% for time spent in the whelping to 85% for licking. For puppy retrieval assay, puppies were grouped in a corner of the whelping box. One puppy was removed from the whelping box and placed in an opposite corner of the whelping room. The dam was given five trials (5 min per trial) a day to approach and retrieve the displaced puppy, in total ten trials in two days (PP2 and PP3) were performed for each dam. The times and latency of approach were analyzed manually by trained experimenters who were blind to the genotype. Approach was defined as a dam walked out of the nursing box to inspect the separated puppy. Retrieval was defined as the dam approached to the separated puppy and then retrieved the puppy to the whelping box. If the dam did not retrieve her puppy within 5 min, the retrieval duration was noted as 5 min.
Intranasal administration of oxytocin
Shank3 mutant dams and WT dams received intranasal OXT at a dose of 40 IU OXT (Bachem, Torrance, USA) dissolved with 100 μl saline or control saline (vehicle) every other day between 9:00–10:00 for two weeks from PP8. A total of 100 μl OXT or vehicle was administered by pipetting 50 μl of solution in each nostril, with 30 seconds between administrations, alternating between the left and right nostril. Animals was placed in a stand position with her head tilted back ~45° with the chin up so the OXT solution could better reach the epithelium. For intranasal administration of oxytocin rescue experiment, dams’ behaviors were recorded from PP8 to PP21 24 h a day. Dams’ behaviors in 60 min video recorded between 6:00–9:00 am on PP9, PP13, PP17 and PP21 for chronic effect after OXT treatment were statistically analyzed. To examine the acute effect of OXT, the maternal behavior was analyzed 15 min after intranasal administration of OXT or vehicle for one hour on PP8. The analysis method for the other days was the same as that on PP1, as described earlier.
Measurements of oxytocin, prolactin and estradiol in blood
Blood samples were collected between 09:00–10:00 from the cephalic vein of the dog. Since hormones such as OXT and prolactin are produced in pulses [2], the whole blood was drawn into disposable medical plain red cap vacuum blood collection tubes (Sanli, Liuyang, China) on PP1 morning during a non-lactating period between 9:00–10:00 after the first meal, to which the protease inhibitor aprotinin (500 IU/μl blood) (Sigma Aldrich, St. Louis, USA) was added immediately. The blood samples were then allowed to clot for 30–60 min at room temperature to prepare serum. The samples were centrifuged at 1600 x g for 15 min at 4 °C. The serum was transferred to a plastic tube and then stored at −80 °C until assay.
OXT concentrations were measured using an OXT ELISA kit (Enzo Life Sciences, Farmingdale, USA). The detection limit of the kit was 15.6 pg/ml. 400 μl serum for each sample was extracted with acetone and petroleum ether according to the product manual and the published method [40]. Extraction is necessary to eliminate interfering substances in the samples and to concentrate OXT for accurate measurements within the range of the assay [40]. The extracted samples were then freeze-dried and reconstituted in 120 μl assay buffer, of which 100 μl was used for the ELISA assay. The reactions were examined with a Thermo microplate reader (Thermo Fisher Scientific, Waltham, USA) and read at 405 nm.
Prolactin in serum was assayed using the Prolactin canine ELISA kit (Demeditec, Kiel, Germany) following the manufacturer’s instructions.
Radioimmunoassay for estradiol was detected by the Beijing North Institute of Biotechnology using an Iodine [125I] Estradiol Radioimmunoassay Kit (Beijing North Institute of Biotechnology, Beijing, China) following the protocol provided by the manufacturer. The minimum level of detection of estradiol was 5 pg/ml. The intra- and inter-assay coefficients of variation were <10% and>
Statistical analysis
All tested samples/animals were included in the analysis. Sample size was not calculated a priori. Before analyzing the differences among different groups, we first analyzed the normality distribution of data. For data that followed a normal distribution, analysis was conducted using independent-samples t test or Pearson correlation. For data that did not follow a normal distribution, analysis was conducted using Mann-Whitney test, Wilcoxon signed rank test or Spearman’s correlation. We used one-way ANOVA and two-way ANOVA to determine statistical significance between three groups. For comparison of multiple groups, we used Bonferroni’s post hoc test. The dotted line represents the 95% confidence interval in Fig. 3D–F. A significant correlation can be considered to exist when p < 0.05 with a correlation coefficient > 0.3 or < −0.3. Calculations were performed with GraphPad Prism 8.0 software. Data are presented as mean ± SEM.
Results
Maternal licking behavior is impaired in Shank3 mutant dams
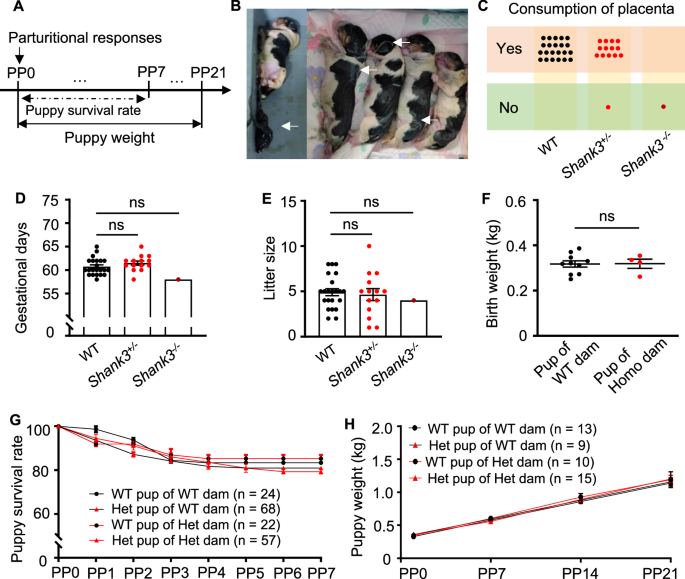
When puppies are born, they can neither see, nor hear, and are unable to regulate their body temperature, for that reason, puppies’ physical and social development is dependent on the interaction with their mothers [4, 5]. From birth to three weeks old, the dam needs to stimulate urination and defecation of puppies through anogenital licking, and provide a heat source to maintain them at a stable body temperature [4, 5, 41]. To examine whether maternal behavior was disrupted in Shank3 mutant dams, we analyzed licking and nursing behaviors together with time stayed in the whelping box quantitatively. We recorded parturitional responses such as licking the amniotic membrane enveloped puppies, biting, and consuming the umbilical cord and placenta immediately after parturition (PP0). Furthermore, we analyzed puppy survival rate from PP0–PP7 (a critical period for survival), and puppy weight gain from PP0–PP21 (Fig. 1A).
A Timeline of parturitional responses, puppy weight, and puppy survival rate analysis. B Puppies were not attended to by the homozygous Shank3 mutant dam Mu210761. The arrows indicated visible placenta and amniotic membranes because the Shank3 homozygous mutant dam did not consume placenta and lick her puppies after delivery. C Shank3 heterozygous mutant dams consumed the placenta. n = 22, 14, and 1 for WT controls, heterozygotes, and homozygote, respectively. D Shank3 mutant dams showed normal gestational days. n = 22, 14, and 1 for WT controls, heterozygotes, and homozygote, respectively. Data are presented as mean ± SEM, ns, no significant differences by one-way ANOVA. E Shank3 mutant dams showed a normal litter size. n = 22, 14, and 1 for WT controls, heterozygotes, and homozygote, respectively. Data are presented as mean ± SEM, ns, no significant differences by one-way ANOVA. F There were no differences in the birth weight of puppies delivered by the Shank3 homozygous mutant dam (pup of Homo dam, red dots, n = 4) compared with WT controls (pup of WT dam, blank dots, n = 10). Data are presented as mean ± SEM. ns, no significant differences by Mann-Whitney test. G There were no significant differences of the postnatal one-week survival rate between 22 WT puppies (WT pup of Het dam) and 57 heterozygous puppies (Het pup of Het dam) of heterozygous Shank3 mutant dams, and 24 WT puppies (WT pup of WT dam) and 68 heterozygous puppies (Het pup of WT dam) of WT dams. ns, no significant differences by two-way ANOVA followed by Bonferroni’s post hoc test. H There were no differences in the weight gain of 10 WT (WT pup of Het dam), 15 heterozygous (Het pup of Het dam) puppies of heterozygous Shank3 mutant dams compared with 13 WT (WT pup of WT dam) and 9 heterozygous (Het pup of WT dam) puppies of WT dams during the first three weeks after birth. All data were presented as mean ± SEM, ns, no significant differences by two-way ANOVA followed by Bonferroni’s post hoc test.
We observed significant maternal behavior defects in one homozygous F2 dam (Mu210761, Table 1). After delivery, she exhibited a lack of consumption of the placenta (Fig. 1B, C) and failed to tear apart the amniotic membranes covering the puppies leading to puppy lethality (Fig. 1B). The puppies were found scattered within the whelping box. However, there were no differences in gestational days (Fig. 1D), as well as litter size (Fig. 1E) and birth weight (Fig. 1F) between the WT dams and the homozygous dam Mu210761. Considering the severe maternal behavior defects in two F0 mutants (Mu180636 carried two small deletions in exon 21; Mu180614 carried a large deletion spanning exons 5 and 21 [8]), we focused our subsequent research solely on the maternal behavior of heterozygous mutant offspring. Unless otherwise specified, the term ‘mutants’ in the following context refers to heterozygous Shank3 mutants; we analyzed maternal behaviors in four heterozygotes of −483+7 bp/+ and seven heterozygotes of −496 bp/+ (Table 1); the two mutations disrupt the Shank3 gene similarly in exon 21 and are transmitted from independent F0 fathers [8]. These heterozygous mutants showed substantially reduced levels of Shank3 isoforms and similar social deficits [8].
Shank3 mutant dams delivered on term (61.50 ± 0.44 days, n = 14 for mutant dams; 60.73 ± 0.38 days, n = 22 for WT. p = 0.4087; Fig. 1D) with a normal litter size (4.64 ± 0.66, n = 14 for mutant dams; 4.91 ± 0.39, n = 22 for WT. p = 0.9653; Fig. 1E). Most Shank3 mutant dams (13/14) consumed the placenta (Fig. 1C). We calculated the survival rate and weight gain of WT and heterozygous puppies of WT dams and mutant dams. There were no significant differences in the survival rate during the first week after birth (Fig. 1G) and weight gain of puppies during the first three weeks after birth (Fig. 1H) of mutant dams compared with WT dams.
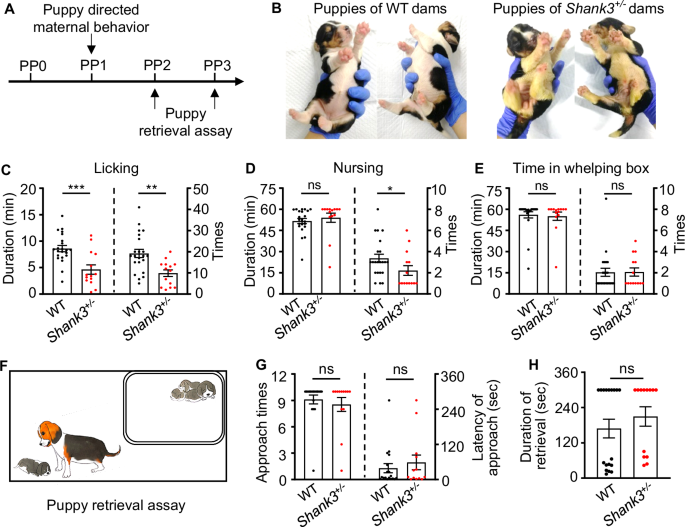
We recorded and analyzed puppy directed maternal behavior of the dam in the whelping box on PP1. Additionally, we analyzed the dam’s reaction to separation of the puppies on PP2 and PP3 (Fig. 2A). We found that Shank3 mutant dams displayed shorter licking duration (278.00 ± 53.64 sec, n = 14 for mutant dams; 8.60 ± 0.59 min, n = 22 for WT. p = 0.0005; Fig. 2C) and fewer times of licking (9.86 ± 1.52, n = 14 for mutant dams; 19.18 ± 1.94, n = 22 for WT. p = 0.0018; Fig. 2C) than the WT dams. This led to some puppies of mutant dams being wet and less clean than the puppies from WT dams (Fig. 2B). The mutant dams showed fewer nursing times (2.16 ± 0.36, n = 14 for mutant dams; 3.41 ± 0.40, n = 22 for WT. p = 0.0380) but a normal nursing duration (54.04 ± 3.25 min, n = 14 for mutant dams; 51.71 ± 1.87 min, n = 22 for WT. p = 0.0534) compared with WT dams (Fig. 2D). We did not find significant differences in duration (55.12 ± 2.94 min, n = 14 for mutant dams; 56.07 ± 2.24 sec, n = 22 for WT. p = 0.5663) and frequency (2.07 ± 0.38, n = 14 for mutant dams; 2.05 ± 0.39, n = 22 for WT. p = 0.8469) of stay in the whelping box between mutant dams and WT dams (Fig. 2E). In addition, we observed no significant differences in the maternal behavior phenotypes between the two genotypes of −483+7 bp/+ and −496 bp/+ (Supplementary Figure S1).
A Timeline of puppy directed maternal behavior and retrieval assay. B The puppies of mutant dams were wet and less clean compared with those reared by WT dams. C Shank3 mutant dams showed a significant reduction in licking duration and times compared with WT dams (n = 14 for mutant dams, n = 22 for WT, Unpaired t test). D The mutant dams showed fewer nursing times but normal nursing duration compared with WT dams (n = 14 for mutant dams, n = 22 for WT, Mann-Whitney test). E The mutant dams did not show significant differences neither in duration nor times of stay in the whelping box (n = 14 for mutant dams, n = 22 for WT controls, Mann-Whitney test). F Illustration of puppy retrieval assay. G There were no significant differences in the approach times or latency to approach the separated puppies between mutant dams and controls (n = 18 for WT, and 14 for mutant dams, by Mann-Whitney test). H There were no significant differences in the duration of retrieval between mutant dams and WT dams (n = 18 for WT, and 14 for mutant dams, by Mann-Whitney test). All data are presented as mean ± SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significant differences.
In the puppy retrieval assay, we evaluated the immediate response of the dam when her puppy was taken out of the whelping box (Fig. 2F). All of WT dams (18/18) and almost all mutant dams (13/14) went out of the whelping box to check on the separated puppies. The retrieval duration for the mutant who stayed in the whelping box for the whole observing time window was noted as 5 min. Compared with WT dams, the Shank3 mutant dams exhibited no significant differences in approach times to the separated puppies (mutant dams: 7.93 ± 0.95, WT: 9.11 ± 0.51, p = 0.4774; Fig. 2G) and in latency to approach the puppies (mutant dams: 75.61 ± 28.78 sec, WT: 38.58 ± 15.64 sec, p = 0.5054; Fig. 2G). In addition, the mutant dams showed no significant differences in duration to retrieve the puppy to the whelping box (mutant dams: 216.10 ± 31.34 sec, WT: 168.70 ± 31.97 sec, p = 0.1642; Fig. 2H).
The maternal licking behavior defects might be associated with social impairments in Shank3 mutant dams. To test this possibility, we analyzed dog-human social interactions of postpartum dams on PP60 after weaning in an open field test as previously described [8]. We found that their social interaction time with a familiar experimenter was significantly lower compared with the WT dams (2.84 ± 0.27 min, n = 9 for WT dams; 1.70 ± 0.40 min, n = 6 for mutant dams. p = 0.0295; Supplementary Figure S2A), consistent with our previous report on non-reproductive adult mutants [8]. Furthermore, there was a significant positive correlation between the dog-human social interaction and maternal licking behavior (n = 15, r = 0.5669, p = 0.0276 for licking duration; and r = 0.5212, p = 0.0463 for licking times; Supplementary Figure S2B, S2C), but no significant correlation between the dog-human social interaction and maternal nursing behavior (n = 15, r = 0.2539, p = 0.3611 for nursing duration; and r = −0.3388, p = 0.2167 for nursing times; Supplementary Figure S2D, S2E).
Shank3 mutant dams exhibit a reduced oxytocin level in the blood
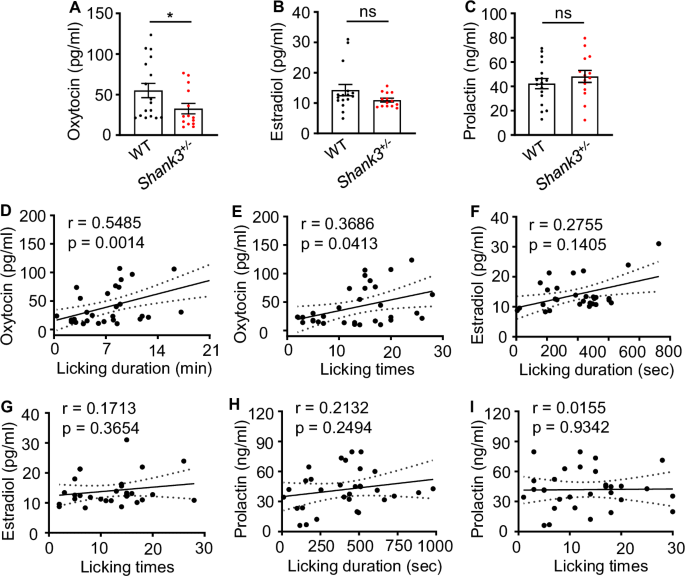
As shown above, the maternal behavior was impaired in Shank3 mutant dams. Hormones such as OXT, prolactin, and sex steroids are involved in the regulation of maternal behavior [16, 42, 43]. To test whether hormones were changed in Shank3 mutant dams, we determined their concentrations in serum collected in the morning of P1 after the first meal, during a non-lactating time window. We found a significant decrease in blood OXT concentrations in Shank3 mutant dams (32.69 ± 6.47 pg/ml, n = 14 for mutant dams; 55.03 ± 8.84 pg/ml, n = 17 for WT. p = 0.0290; Fig. 3A). The mutants also showed significantly lower OXT levels than WT controls in non-reproductive period (20.41 ± 1.08 pg/ml, n = 13 for mutants; 26.47 ± 1.25 pg/ml, n = 10 for WT. p = 0.0014; Supplementary Figure S3). However, we found no significant change in the serum levels of estradiol, the major female sex hormone (10.97 ± 0.62 pg/ml, n = 14 for mutant dams; 14.28 ± 1.86 pg/ml, n = 16 for WT. p = 0.1932; Fig. 3B) and prolactin, which promotes milk production (48.16 ± 4.91 ng/ml, n = 14 for mutant dams; 42.31 ± 4.21 ng/ml, n = 17 for WT. p = 0.3700; Fig. 3C) in Shank3 mutant dams.
A Shank3 mutant dams showed a significantly lower blood OXT level compared with controls on P1. Mann-Whitney test, n = 14 for mutant dams, n = 17 for WT dams. B Shank3 mutant dams displayed no significant differences in blood estradiol level on P1. Mann-Whitney test, n = 14 for mutant dams, n = 16 for WT dams. C Shank3 mutant dams displayed no significant differences in blood prolactin level on P1. Unpaired t test, n = 10 for mutant dams, n = 9 for WT. D, E The blood OXT level was positively correlated with the maternal licking duration D and licking times E. n = 31. F, G No significant correlation between licking duration F or licking times G and the blood estradiol level. n = 30. H and I No significant correlation between licking duration H or licking times I and the prolactin level. n = 19.
To investigate a possible correlation between licking behavior and hormone levels, we analyzed the degree of association. We found a significant positive correlation between licking behavior (n = 31, r = 0.5485, p = 0.0014 for licking duration; Fig. 3D and n = 31, r = 0.3686, p = 0.0413 for licking times; Fig. 3E) and peripheral OXT levels. However, there was no correlation between licking behavior (n = 30, r = 0.2755, p = 0.1405 for licking duration; Fig. 3F and n = 30, r = 0.1713, p = 0.3654 for licking times; Fig. 3G) and peripheral estradiol levels. Similarly, no correlation between licking behavior (n = 31, r = 0.2132, p = 0.2494 for licking duration; Fig. 3H and n = 31, r = 0.0155, p = 0.9342 for licking times; Fig. 3I) and peripheral prolactin levels was observed.
Intranasal oxytocin administration ameliorates maternal behavior defects in Shank3 mutant dams
OXT plays a central role in maternal behavior [20,21,22, 43, 44] and the results shown above indicate that reduced OXT might be responsible for the impaired maternal behavior observed in Shank3 mutant dams. As mentioned earlier, the first week after birth is a critical period for a puppy’s survival, we therefore examined the effect of OXT on maternal behaviors one week after parturition to ensure dam-puppies’ well-being. As the licking behavior of dams towards puppies mainly occurs during the first three weeks after giving birth and declines slowly over time [5], we examined the effects of OXT for a period of 2 weeks starting from PP8. We used 40 IU OXT, which was used previously in pet dogs [45], in the present study.
To determine whether the impaired maternal behavior in Shank3 mutant dams could be improved with intranasal OXT administration, we performed a vehicle-controlled experiment to evaluate the acute (15 min after the first OXT treatment on PP8) and chronic (from PP8 every other day for 2 weeks) effects of intranasal OXT treatment on maternal behaviors in Shank3 mutant dams (Fig. 4A). To increase the sample size (12 mutant dams and 12 WT controls in total), we examined the effects of OXT on maternal behaviors twice after the first and the second parturitions of five animals (four mutant dams and one WT, see Table 1). We quantitatively analyzed the maternal behaviors and found that there were no significant differences in licking, nursing, and time stayed in the whelping box between primiparous and multiparous dams (n = 4; Supplementary Figure S4). Therefore, we concluded that the improvement of maternal behavior in Shank3 mutant dams after intranasal OXT administration was unrelated to whether the dam had experienced single or multiple births.
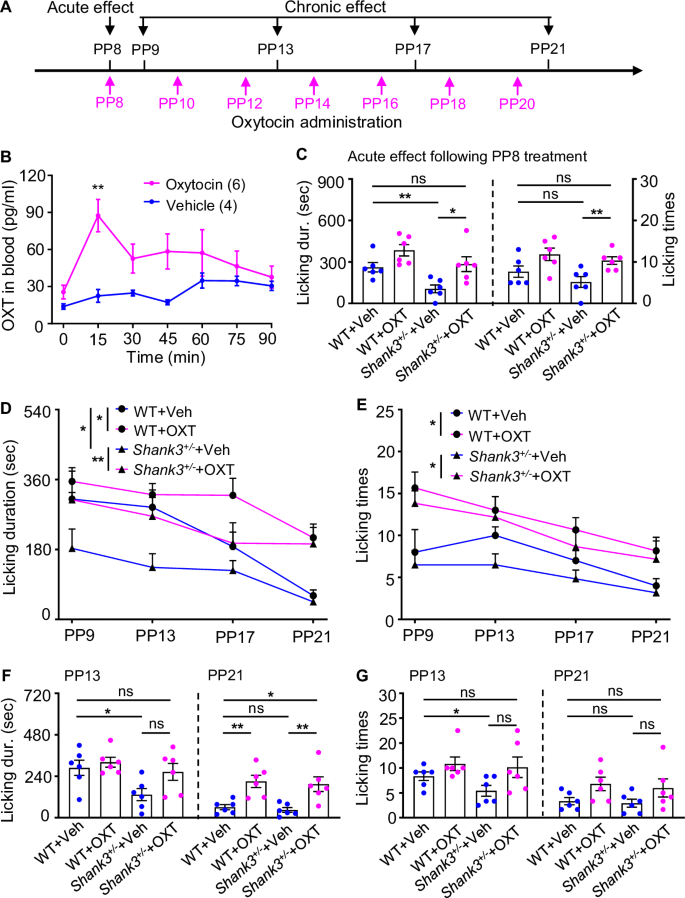
A Timeline of OXT treatment experiment. Arrow indicates the time point of OXT or vehicle administration. B Blood OXT concentrations (mean ± SEM) at baseline (0 min) and every 15 min after intranasal administration of OXT (pink, n = 6) or vehicle (blue, n = 4). **p < 0.01 compared with baseline level by Wilcoxon signed rank test. C Quantitative assessment of maternal licking duration (left) and times (right) in dams 15 min after a single dose of OXT treatment versus vehicle on PP8. n = 6 for each condition, WT dams treated with vehicle (WT+Veh) or OXT (WT + OXT), mutant dams treated with vehicle (Shank3+/−+Veh) or OXT (Shank3+/−+OXT). All error bars are SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significant differences by two-way ANOVA followed by Bonferroni’s post hoc test. D, E Mean licking duration D and times E of WT and Shank3 mutant dams treated with vehicle or OXT. F, G Quantitative assessment of maternal licking duration F and times G in dams after one (PP13) and two (PP21) weeks of OXT versus vehicle treatment. n = 6 for each condition. All error bars are SEM. *p < 0.05; **p < 0.01; ***p < 0.001; ns, no significant differences by two-way ANOVA followed by Bonferroni’s post hoc test.
To verify the effectiveness of exogenous OXT, we examined the OXT levels in blood at different time points after OXT administration. Compared with vehicle, blood OXT levels were significantly increased 15 min after intranasal OXT administration (87.47 ± 13.08 pg/ml, n = 6 for OXT treatment; 22.49 ± 5.24 pg/ml, n = 4 for vehicle treatment. p = 0.0095.) and returned to baseline levels approximately 90 min later (Fig. 4B), demonstrating the efficacy of OXT administration. The blood level dynamics of OXT after intranasal administration was in agreement with a previous report in pet dogs [46].
To assess the acute effects, maternal behavior was analyzed 15 min after administration on PP8. After OXT treatment, the duration and times of licking in the mutant dams were increased significantly. The mutant dams treated with vehicle (Shank3+/−+Veh, n = 6) exhibited significantly lower licking duration compared with the WT dams treated with vehicle (WT+Veh, n = 6) (Shank3+/−+Veh: 105.20 ± 28.35 sec, WT+Veh: 261.50 ± 33.76 sec, p = 0.0043; Fig. 4C). The duration of licking behavior was significantly longer in the mutant dams treated with OXT (Shank3+/−+OXT, n = 6), reaching the level of the WT+Veh dams (Shank3+/−+OXT: 284.20 ± 53.85 sec, WT+Veh: 261.50 ± 33.76 sec, p = 0.5887; Fig. 4C). It also showed no significant differences when compared with the WT dam treated with OXT (WT + OXT, n = 6) (Shank3+/−+OXT: 284.20 ± 53.85 sec, WT + OXT: 384.50 ± 41.28 sec, p = 0.0931; Fig. 4C). Shank3+/−+OXT dams also showed significantly increased licking times compared with Shank3+/−+Veh dams (Shank3+/−+OXT: 10.33 ± 0.92, Shank3+/−+Veh: 5.17 ± 1.30, p = 0.0065; Fig. 4C). Thus, there was a significant acute improvement of Shank3 mutant dams’ licking behavior after the administration of one dose of OXT.
To determine the chronic effects of two-week administration of OXT on maternal behavior, we analyzed the maternal behavior on PP9, PP13, PP17 and PP21. For both WT and mutant dams across the whole test period, the licking duration (Fig. 4D) and times (Fig. 4E) tended to decrease over time as the puppies grew. After intranasal OXT treatment, the duration (p = 0.0060; Fig. 4D) and times (p = 0.006; Fig. 4C) of licking in the mutant dams were increased significantly. The licking behavior of Shank3+/−+Veh dams was significantly lower than that of WT+Veh dams (p = 0.032; Fig. 4D). The differences between the two groups were reduced when OXT was administered (p = 0.134; Fig. 4D). It is worth noting that OXT administration also increased the maternal licking behavior of WT dams, as evidenced by a significant increase in licking duration (p = 0.031; Fig. 4D) and licking frequency (p = 0.011; Fig. 4E).
Within this two-week OXT treatment period, we specifically analyzed the effects of OXT treatment at two representative time points: at one week (PP13) and two weeks (PP21) after OXT administration. Similar to the effects of acute administration, the duration of licking behavior was significantly shorter in the Shank3+/−+Veh dams compared with the WT+Veh dams (Shank3+/−+Veh: 133.70 ± 35.07 sec, WT+Veh: 288.67 ± 44.46 sec, p = 0.0411; Fig. 4F), Shank3 mutant dams showed increased licking duration, and reached the level of the WT+Veh dams (Shank3+/−+OXT: 265.30 ± 48.73 sec, WT+Veh: 288.67 ± 44.46 sec, p = 0.9372) after one week of OXT treatment (Fig. 4F). The Shank3+/−+Veh dams exhibited significantly lower licking times when compared with the WT+Veh dams (Shank3+/−+Veh: 6.50 ± 1.31, WT+Veh: 10.00 ± 1.03, p = 0.0433; Fig. 4G). However, Shank3+/−+OXT dams showed increased licking times, reaching the level of WT+Veh dams (Shank3+/−+OXT: 12.17 ± 2.47, WT+Veh: 10.00 ± 1.03, p = 0.9242; Fig. 4G). After two weeks of OXT administration, the Shank3+/−+OXT dams exhibited a significantly longer licking duration when compared with the Shank3+/−+Veh dams (Shank3+/−+OXT: 194.20 ± 42.28 sec, Shank3+/−+Veh: 45.17 ± 14.13 sec, p = 0.0087; Fig. 4F), which was significantly longer than the WT+Veh dams (Shank3+/−+OXT: 194.20 ± 42.28 sec, WT+Veh: 61.00 ± 14.97 sec, p = 0.0130; Fig. 4F). After two weeks, the licking duration also significantly increased in the WT + OXT dams compared with the WT+Veh dams (WT + OXT: 210.33 ± 35.10 sec, WT+Veh: 61.00 ± 14.97 sec, p = 0.0065; Fig. 4F).
In conclusion, both acute single dose and chronic multiple intranasal OXT administrations every other day for two weeks were able to rescue the decreased licking behavior in Shank3 mutant dams. Specifically, the licking duration and times of the Shank3 mutant dams significantly increased after OXT treatment, reaching levels similar to those of WT dams. Intranasal OXT also increased licking behavior in the WT dogs. These results support a role for OXT in promoting maternal behavior.
Discussion
Little is known about the underlying mechanisms of maternal behavior deficits in Shank3 mutant dams. We found that blood OXT levels in Shank3 mutant dams was significantly lower than those in the WT dams, and there was a significant positive correlation between OXT levels and maternal behavior. Furthermore, we observed that the reduced maternal behavior in Shank3 mutant dams was rescued by OXT. These findings, to the best of our knowledge, have never been documented in any ASD animal models. It has been suggested that deficits of the OXT system may underlie the social impairments in ASD [47,48,49]. It remains to be determined whether a reduced level of OXT contributes to the impaired social interaction including maternal deficits in, at least, a subset of ASD patients.
There are few studies on maternal behavior in ASD animal models. One study reported that loss of Shank2 in mice leads to a lack of maternal behavior but unaltered OXT, estradiol, or prolactin levels in the blood [17]. Shank2-/- mice display reduced neuronal activity detected by c-Fos staining in the nuclei of the social attachment circuit that includes the medial preoptic area (MPOA) of the hypothalamus; selective enhancement of MPOA activity by chemogenetics re-established maternal behavior in Shank2−/− mice [17]. In the present study, we found that peripheral levels of OXT were significantly lower in Shank3 mutant dams, while other hormones such as prolactin and estradiol showed no abnormalities. Shank3 mutations appear to selectively affect the OXT system and OXT administration can rescue the maternal behavior defects in Shank3 mutant dams.
There are a few genes that regulate OXT, directly or indirectly, at different steps. For example, cluster of differentiation 38 (CD38) is a transmembrane protein that promotes Ca2+ elevation and depolarization-induced OXT secretion in oxytocinergic axon terminals [50]. CD38−/− mice show reduced OXT levels in plasma and apparent defects in maternal nurturing behavior [50]. The loss of function of the Fmr1 gene (which encodes the fragile X mental retardation protein) induced a decreased staining intensity of OXT in the PVN [51, 52]. In the Fmr1−/− brain, CA1 neurons exhibit an excessive response to incoming excitatory signals, and in turn exert enhanced inhibition to the PVN, potentially suppressing OXT production [51]. Mice lacking the contactin-associated protein-like 2 gene (Cntnap2), exhibit abnormalities in social behavior, as well as fewer OXT-positive neurons and a decreased intensity of OXT staining in the PVN [53]. Acute administration of OXT improved social deficits, and chronic early postnatal treatment with OXT led to more lasting behavioral recovery and restored OXT immunoreactivity in the PVN in Cntnap2−/− mice [53]. An autism-associated mutation in the synaptic adhesion molecule Nlgn3 results in an impaired response to OXT in dopaminergic neurons and altered behaviors in social novelty text [54]. How these ASD risk genes mechanistically affect the OXT pathway remains to be elucidated.
Although over thirty animal models with various Shank3 mutations have been generated [10], there is relatively limited research on the relationship between Shank3 and OXT [55,56,57,58,59]. Although Shank3−/− rats exhibited normal central OXT levels by immunostaining and mRNA quantification [57], OXT was reduced in other Shank3 mutant animals including mutant dogs described in the present study, while one study reported a reduction in OXT positive neurons in Shank3B−/− mice, and that acute intranasal OXT can rescue social deficits [58]. These discrepancies may be attributed to species-specific biology, diverse genetic backgrounds, and different targeting sites in the Shank3 gene. Acute OXT application improved social memory, attention, and synaptic plasticity deficits in Shank3+/− and Shank3−/− rats [55]. Acute OXT also restored abnormal neuronal morphology in primary hippocampal neurons of Shank3−/− mice and compensated for the altered synaptic proteins in a human neuron-like cell line with the SHANK3 gene silenced by small interference RNAs [59]. Chronic intranasal OXT administration can reliably ameliorate social deficits in the Shank3B−/− mice as early as 2 weeks after daily treatment, though the OXT levels in the mutants were not reported [56]. The results of acute and chronic oxytocin treatment appeared similar in rescuing the licking behavior deficits of Shank3 mutants. We speculate that even though exogenous oxytocin is rapidly metabolized in the body, it may affect relevant neural pathways and induce long lasting effects on promoting social behaviors such as maternal licking. The mechanisms underlying the rescuing effect of oxytocin remain to be clarified. In addition to administration of exogenous OXT, there are other methods including social touch [60], chemogenetics [53, 60], and optogenetics [61] to increase endogenous OXT signaling. Given the improvement of maternal behavior in Shank3 mutant mice and dogs by OXT, it is possible that patients with SHANK3 mutations might potentially benefit from endogenous and/or exogenous OXT intervention.
The aforementioned studies indicate that Shank3 mutations can lead to deficits in the OXT system. However, our own study as well as previous studies have not investigated the underlying mechanisms. We have yet to determine whether the decrease in OXT levels in the peripheral blood of Shank3 mutant dogs is due to an effect on OXT-positive neurogenesis, OXT synthesis or secretion. Future studies should determine whether Shank3 mutation can affect the upstream pathways that regulate OXT production and secretion or whether it also perturbs pathways further downstream. Shank3 mutant dams exhibited a significant decrease in maternal behavior, but no impact on puppies’ survival rate and weight gain. As maternal behaviors may further affect the puppies’ cognition and social behaviors [4, 5, 39], how Shank3 mutations in dams affect their offspring’s social behaviors remains to be elucidated in the future studies.
Data availability
All relevant analyses for this paper are described in the manuscript. Data supporting the findings are available from the corresponding authors upon request.
References
-
Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900.
-
Kohl J, Autry AE, Dulac C. The neurobiology of parenting: a neural circuit perspective. Bioessays. 2017;39:1–11.
-
Dulac C, O’Connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–70.
-
Lezama-Garcia K, Mariti C, Mota-Rojas D, Martinez-Burnes J, Barrios-Garcia H, Gazzano A. Maternal behaviour in domestic dogs. Int J Vet Sci Med. 2019;7:20–30.
-
Santos NR, Beck A, Fontbonne A. A review of maternal behaviour in dogs and potential areas for further research. J Small Anim Pract. 2020;61:85–92.
-
Association Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Arlington, VA: American Psychiatric Association; 2013. p. 947.
-
Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–63.
-
Tian R, Li Y, Zhao H, Lyu W, Zhao J, Wang X, et al. Modeling SHANK3-associated autism spectrum disorder in Beagle dogs via CRISPR/Cas9 gene editing. Mol Psychiatry. 2023;28:3739–50.
-
Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27.
-
Delling JP, Boeckers TM. Comparison of SHANK3 deficiency in animal models: phenotypes, treatment strategies, and translational implications. J Neurodev Disord. 2021;13:55.
-
Zhou Y, Sharma J, Ke Q, Landman R, Yuan J, Chen H, et al. Atypical behaviour and connectivity in SHANK3-mutant macaques. Nature. 2019;570:326–31.
-
Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci. 2015;16:551–63.
-
Gardner M, Suplee PD, Bloch J, Lecks K. Exploratory Study of Childbearing Experiences of Women With Asperger Syndrome. Nurs Womens Health. 2016;20:28–37.
-
Rogers C, Lepherd L, Ganguly R, Jacob-Rogers S. Perinatal issues for women with high functioning autism spectrum disorder. Women Birth. 2017;30:e89–e95.
-
Pohl AL, Crockford SK, Blakemore M, Allison C, Baron-Cohen S. A comparative study of autistic and non-autistic women’s experience of motherhood. Mol Autism. 2020;11:3.
-
Bridges RS. Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol. 2015;36:178–96.
-
Grabrucker S, Pagano J, Schweizer J, Urrutia‐Ruiz C, Schön M, Thome K, et al. Activation of the medial preoptic area (MPOA) ameliorates loss of maternal behavior in a Shank2 mouse model for autism. The EMBO J. 2021;40:e104267.
-
Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev. 2018;98:1805–908.
-
Li H, Jiang T, An S, Xu M, Gou L, Ren B, et al. Single-neuron projectomes of mouse paraventricular hypothalamic nucleus oxytocin neurons reveal mutually exclusive projection patterns. Neuron. 2024;112:1081–99.e7.
-
Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr. Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–50.
-
Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46:56–61.
-
Carcea I, Caraballo NL, Marlin BJ, Ooyama R, Riceberg JS, Mendoza Navarro JM, et al. Oxytocin neurons enable social transmission of maternal behaviour. Nature. 2021;596:553–7.
-
Valtcheva S, Issa HA, Bair-Marshall CJ, Martin KA, Jung K, Zhang Y, et al. Neural circuitry for maternal oxytocin release induced by infant cries. Nature. 2023;621:788–95.
-
Pedersen CA, Vadlamudi SV, Boccia ML, Amico JA. Maternal behavior deficits in nulliparous oxytocin knockout mice. Genes Brain Behav. 2006;5:274–81.
-
Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–101.
-
Pobbe RL, Pearson BL, Blanchard DC, Blanchard RJ. Oxytocin receptor and Mecp2 308/Y knockout mice exhibit altered expression of autism-related social behaviors. Physiol Behav. 2012;107:641–8.
-
Higashida H, Lopatina O, Yoshihara T, Pichugina YA, Soumarokov AA, Munesue T, et al. Oxytocin signal and social behaviour: comparison among adult and infant oxytocin, oxytocin receptor and CD38 gene knockout mice. J Neuroendocrinol. 2010;22:373–9.
-
Horie K, Inoue K, Suzuki S, Adachi S, Yada S, Hirayama T, et al. Oxytocin receptor knockout prairie voles generated by CRISPR/Cas9 editing show reduced preference for social novelty and exaggerated repetitive behaviors. Horm Behav. 2019;111:60–9.
-
Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta). Proc Natl Acad Sci USA. 2012;109:959–64.
-
Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–8.
-
Menon R, Neumann ID. Detection, processing and reinforcement of social cues: regulation by the oxytocin system. Nat Rev Neurosci. 2023;24:761–77.
-
Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–71.
-
Watanabe T, Kuroda M, Kuwabara H, Aoki Y, Iwashiro N, Tatsunobu N, et al. Clinical and neural effects of six-week administration of oxytocin on core symptoms of autism. Brain. 2015;138:3400–12.
-
Fastman J, Foss-Feig J, Frank Y, Halpern D, Harony-Nicolas H, Layton C, et al. A randomized controlled trial of intranasal oxytocin in Phelan-McDermid syndrome. Mol Autism. 2021;12:62.
-
Guastella AJ, Hickie IB. Oxytocin treatment, circuitry, and autism: a critical review of the literature placing oxytocin into the autism context. Biol Psychiatry. 2016;79:234–42.
-
Sikich L, Kolevzon A, King BH, McDougle CJ, Sanders KB, Kim SJ, et al. Intranasal oxytocin in children and adolescents with autism spectrum disorder. N Engl J Med. 2021;385:1462–73.
-
Guardini G, Bowen J, Raviglione S, Farina R. Maternal behaviour in domestic dogs: a comparison between primiparous and multiparous dogs. Dog Behavior. 2015;1:23–33.
-
Czerwinski VH, Smith BP, Hynd PI, Hazel SJ. Sampling maternal care behaviour in domestic dogs: What’s the best approach? Behav processes. 2017;140:41–46.
-
Foyer P, Wilsson E, Jensen P. Levels of maternal care in dogs affect adult offspring temperament. Sci Rep. 2016;6:19253.
-
Zhang HF, Dai YC, Wu J, Jia MX, Zhang JS, Shou XJ, et al. Plasma oxytocin and arginine-vasopressin levels in children with autism spectrum disorder in china: associations with symptoms. Neurosci Bull. 2016;32:423–32.
-
Bray EE, Sammel MD, Cheney DL, Serpell JA, Seyfarth RM. Characterizing early maternal style in a population of guide dogs. Front Psychol. 2017;8:175.
-
Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci USA. 1990;87:8003–7.
-
Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–6.
-
Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC. Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature. 2015;520:499–504.
-
Nagasawa M, Mitsui S, En S, Ohtani N, Ohta M, Sakuma Y, et al. Social evolution. Oxytocin-gaze positive loop and the coevolution of human-dog bonds. Science. 2015;348:333–6.
-
Romero T, Nagasawa M, Mogi K, Hasegawa T, Kikusui T. Oxytocin promotes social bonding in dogs. Proc Natl Acad Sci USA. 2014;111:9085–90.
-
Parker KJ, Garner JP, Libove RA, Hyde SA, Hornbeak KB, Carson DS, et al. Plasma oxytocin concentrations and OXTR polymorphisms predict social impairments in children with and without autism spectrum disorder. Proc Natl Acad Sci USA. 2014;111:12258–63.
-
Neuhaus E, Beauchaine TP, Bernier R. Neurobiological correlates of social functioning in autism. Clin Psychol Rev. 2010;30:733–48.
-
Dolen G. Autism: Oxytocin, serotonin, and social reward. Soc Neurosci. 2015;10:450–65.
-
Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–5.
-
Marsillo A, David L, Gerges B, Kerr D, Sadek R, Lasiychuk V, et al. PKC epsilon as a neonatal target to correct FXS-linked AMPA receptor translocation in the hippocampus, boost PVN oxytocin expression, and normalize adult behavior in Fmr1 knockout mice. Biochim Biophys Acta Mol Basis Dis. 2021;1867:166048.
-
Francis SM, Sagar A, Levin-Decanini T, Liu W, Carter CS, Jacob S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014;1580:199–218.
-
Peñagarikano O, Lázaro MT, Lu XH, Gordon A, Dong H, Lam HA, et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci Transl Med. 2015;7:271ra278.
-
Hörnberg H, Perez-Garci E, Schreiner D, Hatstatt-Burkle L, Magara F, Baudouin S, et al. Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature. 2020;584:252–6.
-
Harony-Nicolas H, Kay M, du Hoffmann J, Klein ME, Bozdagi-Gunal O, Riad M, et al. Oxytocin improves behavioral and electrophysiological deficits in a novel Shank3-deficient rat. Elife. 2017;6:e18904.
-
Szabó J, Mlynár M, Feješ A, Renczés E, Borbélyová V, Ostatníková D, et al. Intranasal oxytocin in a genetic animal model of autism. Mol Psychiatry. 2024;29:342–7.
-
Song TJ, Lan XY, Wei MP, Zhai FJ, Boeckers TM, Wang JN, et al. Altered behaviors and impaired synaptic function in a novel rat model with a complete Shank3 deletion. Front Cell Neurosci. 2019;13:111.
-
Sgritta M, Dooling SW, Buffington SA, Momin EN, Francis MB, Britton RA, et al. Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101:246–59.e246.
-
Reichova A, Bacova Z, Bukatova S, Kokavcova M, Meliskova V, Frimmel K, et al. Abnormal neuronal morphology and altered synaptic proteins are restored by oxytocin in autism-related SHANK3 deficient model. Mol Cell Endocrinol. 2020;518:110924.
-
Yu H, Miao W, Ji E, Huang S, Jin S, Zhu X, et al. Social touch-like tactile stimulation activates a tachykinin 1-oxytocin pathway to promote social interactions. Neuron. 2022;110:1051–67.e1057.
-
Liu Y, Li A, Bair-Marshall C, Xu H, Jee HJ, Zhu E, et al. Oxytocin promotes prefrontal population activity via the PVN-PFC pathway to regulate pain. Neuron. 2023;111:1795–811.e1797.
Acknowledgements
We thank Prof. Zhiheng Xu, Qing-feng Wu, and Hui Zhao for discussion. We thank Dr. Ruiqi Shao for drawing the illustration of puppy retrieval assay. This work was supported in part by the Ministry of Science and Technology of China (2021ZD0203901), Spring City Plan (2022SCP001) and the National Natural Science Foundation of China (32394030).
Author information
Authors and Affiliations
Contributions
YQZ conceptualized the project, supervised data collection and analysis. WL, YL, RZ, JPZ and RT designed experiments. WL, YL and QQT performed behavioral experiments. WL and YL assisted data collection and analysis. WL wrote the manuscript. AYY, KG, YHJ, and YQZ finalized the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All animal-related protocols, as well as animal care and handling, were approved in advance by the Institutional Animal Care and Use Committee of Institute of Genetics and Developmental Biology (AP2024026).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lyu, W., Li, Y., Yao, A. et al. Oxytocin improves maternal licking behavior deficits in autism-associated Shank3 mutant dogs.
Transl Psychiatry 15, 76 (2025). https://doi.org/10.1038/s41398-025-03296-5
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41398-025-03296-5
This post was originally published on this site be sure to check out more of their content.