Abstract
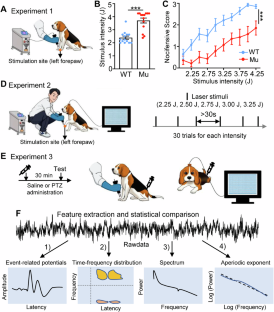
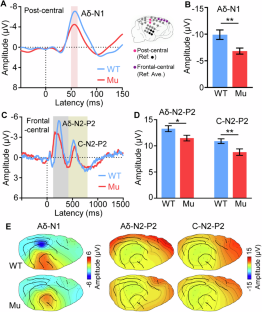
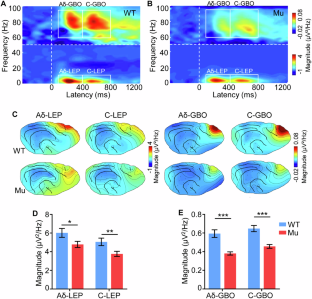
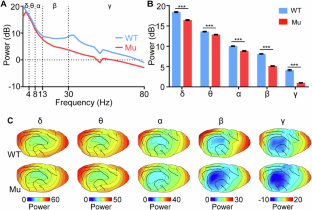
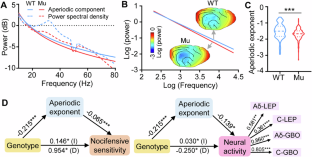
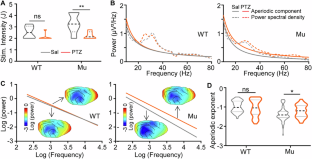
Autistic individuals carrying mutations in SHANK3 (encoding a synaptic scaffolding protein) have been consistently reported to exhibit reduced pain sensitivity. However, the neural mechanisms underlying impaired pain processing remain unclear. To investigate the role of SHANK3 in pain processing, we conducted behavioral, electrophysiological, and pharmacological tests upon nociceptive stimulation in a Shank3 mutant dog model. Behaviorally, Shank3 mutant dogs showed reduced nocifensive sensitivity compared to wild-type (WT) dogs. Electrophysiologically, Shank3 mutant dogs exhibited reduced neural responses elicited by the activations of both Aδ- and C-fiber nociceptors. Additionally, Shank3 mutants showed a lower level of aperiodic exponents, which serve as a marker for the excitatory-inhibitory balance of neural activity. The aperiodic exponents mediated the relationship between genotype and nocifensive sensitivity as well as between genotype and neural responses elicited by nociceptive stimuli. Pharmacologically, the reduced nocifensive sensitivity and atypical excitatory-inhibitory balance were rescued by a GABAAR antagonist pentylenetetrazole. These findings highlight the critical role of Shank3 in pain processing and suggest that an impaired excitatory-inhibitory balance may be responsible for the reduced nocifensive reactivity in autism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout















Data availability
The data and analysis code that support the findings of this study are available from the corresponding authors upon reasonable request.
References
-
Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. 2020;161:1976–82.
-
Guan J, Li G. Injury mortality in individuals with autism. Am J Public Health. 2017;107:791–3.
-
Allely CS. Pain sensitivity and observer perception of pain in individuals with autistic spectrum disorder. ScientificWorldJournal. 2013;2013:e916178.
-
Moore DJ. Acute pain experience in individuals with autism spectrum disorders: a review. Autism. 2015;19:387–99.
-
Furniss F, Biswas AB. Recent research on aetiology, development and phenomenology of self-injurious behaviour in people with intellectual disabilities: a systematic review and implications for treatment. J Intellect Disabil Res. 2012;56:453–75.
-
Chien YL, Wu SW, Chu CP, Hsieh ST, Chao CC, Gau SSF. Attenuated contact heat-evoked potentials associated with sensory and social-emotional symptoms in individuals with autism spectrum disorder. Sci Rep. 2017;7:36887.
-
Failla MD, Moana-Filho EJ, Essick GK, Baranek GT, Rogers BP, Cascio CJ. Initially intact neural responses to pain in autism are diminished during sustained pain. Autism. 2018;22:669–83.
-
Rubinstein M, Patowary A, Stanaway IB, McCord E, Nesbitt RR, Archer M, et al. Association of rare missense variants in the second intracellular loop of NaV1.7 sodium channels with familial autism. Mol Psychiatry. 2018;23:231–9.
-
Oberman LM, Boccuto L, Cascio L, Sarasua S, Kaufmann WE. Autism spectrum disorder in Phelan-McDermid syndrome: initial characterization and genotype-phenotype correlations. Orphanet J Rare Dis. 2015;10:105.
-
Yuan B, Wang M, Wu X, Cheng P, Zhang R, Zhang R, et al. Identification of de novo mutations in the chinese autism spectrum disorder cohort via whole-exome sequencing unveils brain regions implicated in autism. Neurosci Bull. 2023;39:1469–80.
-
Monteiro P, Feng G. SHANK proteins: roles at the synapse and in autism spectrum disorder. Nat Rev Neurosci. 2017;18:147–57.
-
Sarasua SM, Boccuto L, Sharp JL, Dwivedi A, Chen C-F, Rollins JD, et al. Clinical and genomic evaluation of 201 patients with Phelan–McDermid syndrome. Hum Genet. 2014;133:847–59.
-
Tavassoli T, Layton C, Levy T, Rowe M, George-Jones J, Zweifach J, et al. Sensory reactivity phenotype in Phelan-Mcdermid syndrome is distinct from idiopathic ASD. Genes. 2021;12:977.
-
Ko HG, Oh SB, Zhuo M, Kaang BK. Reduced acute nociception and chronic pain in Shank2-/- mice. Mol Pain. 2016;12:1744806916647056.
-
Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–67.
-
Wang L, Almeida LEF, Nettleton M, Khaibullina A, Albani S, Kamimura S, et al. Altered nocifensive behavior in animal models of autism spectrum disorder: the role of the nicotinic cholinergic system. Neuropharmacology. 2016;111:323–34.
-
Chen Y, Yu J, Niu Y, Qin D, Liu H, Li G, et al. Modeling rett syndrome using TALEN-edited MECP2 mutant cynomolgus monkeys. Cell. 2017;169:945–55.
-
Han Q, Kim YH, Wang X, Liu D, Zhang ZJ, Bey AL, et al. SHANK3 deficiency impairs heat hyperalgesia and TRPV1 signaling in primary sensory neurons. Neuron. 2016;92:1279–93.
-
Bunford N, Andics A, Kis A, Miklósi Á, Gácsi M. Canis familiaris as a model for non-invasive comparative neuroscience. Trends Neurosci. 2017;40:438–52.
-
Topál J, Román V, Turcsán B. The dog (Canis familiaris) as a translational model of autism: it is high time we move from promise to reality. Wiley Interdiscip Rev Cogn Sci. 2019;10:e1495.
-
Czeibert K, Andics A, Petneházy Ö, Kubinyi E. A detailed canine brain label map for neuroimaging analysis. Biol Futur. 2019;70:112–20.
-
Hernandez-Avalos I, Mota-Rojas D, Mora-Medina P, Martínez-Burnes J, Casas Alvarado A, Verduzco-Mendoza A, et al. Review of different methods used for clinical recognition and assessment of pain in dogs and cats. Int J Vet Sci Med. 2019;7:43–54.
-
van Oostrom H, Stienen PJ, Doornenbal A, Hellebrekers LJ. Nociception-related somatosensory evoked potentials in awake dogs recorded after intra epidermal electrical stimulation. Eur J Pain. 2009;13:154–60.
-
Rutherford KMD. Assessing pain in animals. Anim Welf. 2002;11:31–53.
-
Barry RJ, De Blasio FM. Characterizing pink and white noise in the human electroencephalogram. J Neural Eng. 2021;18:034001.
-
Donoghue T, Watrous AJ. How can we differentiate narrow-band oscillations from aperiodic activity? Intracranial EEG: A Guide for Cognitive Neuroscientists. Cham: Springer International Publishing; 2023. p. 351–64.
-
Ahmad J, Ellis C, Leech R, Voytek B, Garces P, Jones E, et al. From mechanisms to markers: novel noninvasive EEG proxy markers of the neural excitation and inhibition system in humans. Transl Psychiatry. 2022;12:467.
-
Peirs C, Seal RP. Neural circuits for pain: recent advances and current views. Science. 2016;354:578–84.
-
Torsney C, MacDermott AB. Disinhibition opens the gate to pathological pain signaling in superficial neurokinin 1 receptor-expressing neurons in rat spinal cord. J Neurosci. 2006;26:1833–43.
-
Xiong W, Ping X, Ripsch MS, Chavez GSC, Hannon HE, Jiang K, et al. Enhancing excitatory activity of somatosensory cortex alleviates neuropathic pain through regulating homeostatic plasticity. Sci Rep. 2017;7:12743.
-
Han Q, Wang H, Lu X, Li Y, Guo Y, Zhao X, et al. Preoperative resting-state electrophysiological signals predict acute but not chronic postoperative pain. Eur J Pain. 2025;29:e4757.
-
Chini M, Pfeffer T, Hanganu-Opatz I. An increase of inhibition drives the developmental decorrelation of neural activity. eLife. 2022;11:e78811.
-
Gao R, Peterson EJ, Voytek B. Inferring synaptic excitation/inhibition balance from field potentials. Neuroimage. 2017;158:70–78.
-
Lombardi F, Herrmann HJ, de Arcangelis L. Balance of excitation and inhibition determines 1/f power spectrum in neuronal networks. Chaos. 2017;27:047402.
-
Lendner JD, Helfrich RF, Mander BA, Romundstad L, Lin JJ, Walker MP, et al. An electrophysiological marker of arousal level in humans. eLife. 2020;9:e55092.
-
Gonzalez-Burgos I, Bainier M, Gross S, Schoenenberger P, Ochoa JA, Valencia M, et al. Glutamatergic and GABAergic receptor modulation present unique electrophysiological fingerprints in a concentration-dependent and region-specific manner. eNeuro. 2023;10:ENEURO.0406-22.2023.
-
Potter LE, Paylor JW, Suh JS, Tenorio G, Caliaperumal J, Colbourne F, et al. Altered excitatory-inhibitory balance within somatosensory cortex is associated with enhanced plasticity and pain sensitivity in a mouse model of multiple sclerosis. J Neuroinflammation. 2016;13:142.
-
Tian R, Li Y, Zhao H, Lyu W, Zhao J, Wang X, et al. Modeling Shank3-associated autism spectrum disorder in beagle dogs via CRISPR/Cas9 gene editing. Mol Psychiatry. 2023;28:3739–50.
-
Ren W, Yu S, Guo K, Lu C, Zhang YQ. Disrupted human-dog interbrain neural coupling in autism-associated Shank3 mutant dogs. Adv Sci. 2024;11:e2402493.
-
Shi Q, Wu L, Ren B, Guo K, Jiang Y-H, Zhang YQ, et al. Impaired tactile processing in autism-associated Shank3 mutant dogs: neural mechanism and intervention. Sci Bull. 2025;70:483–7.
-
Bromm B, Treede RD. Laser-evoked cerebral potentials in the assessment of cutaneous pain sensitivity in normal subjects and patients. Rev Neurol. 1991;147:625–43.
-
Carmon A, Mor J, Goldberg J. Evoked cerebral responses to noxious thermal stimuli in humans. Exp Brain Res. 1976;25:103–7.
-
Leandri M, Saturno M, Spadavecchia L, Iannetti GD, Cruccu G, Truini A. Measurement of skin temperature after infrared laser stimulation. Neurophysiol Clin. 2006;36:207–18.
-
Wu L, Mei S, Yu S, Han S, Zhang YQ. Shank3 mutations enhance early neural responses to deviant tones in dogs. Cereb Cortex. 2023;33:10546–57.
-
Hu L, Cai MM, Xiao P, Luo F, Iannetti GD. Human brain responses to concomitant stimulation of Aδ and C nociceptors. J Neurosci. 2014;34:11439–51.
-
Hu L, Zhang Z. EEG signal processing and feature extraction. Singapore: Springer Singapore; 2019.
-
Hu L, Iannetti GD. Neural indicators of perceptual variability of pain across species. Proc Natl Acad Sci USA. 2019;116:1782–91.
-
Mitsis GD, Iannetti GD, Smart TS, Tracey I, Wise RG. Regions of interest analysis in pharmacological fMRI: How do the definition criteria influence the inferred result? Neuroimage. 2008;40:121–32.
-
Donoghue T, Haller M, Peterson EJ, Varma P, Sebastian P, Gao R, et al. Parameterizing neural power spectra into periodic and aperiodic components. Nat Neurosci. 2020;23:1655–65.
-
Huang RQ, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, Dillon GH. Pentylenetetrazole-Induced inhibition of recombinant γ-Aminobutyric acid Type A (GABAA) receptors: mechanism and site of action. J Pharmacol Exp Ther. 2001;298:986–95.
-
Wu X, Lu X, Zhang H, Bi Y, Gu R, Kong Y, et al. Sex difference in trait empathy is encoded in the human anterior insula. Cereb Cortex. 2023;33:5055–65.
-
Zhang H, Bi Y, Hou X, Lu X, Tu Y, Hu L. The role of negative emotions in sex differences in pain sensitivity. Neuroimage. 2021;245:118685.
-
Tu Y, Zhang Z, Tan A, Peng W, Hung YS, Moayedi M, et al. Alpha and gamma oscillation amplitudes synergistically predict the perception of forthcoming nociceptive stimuli. Hum Brain Mapp. 2016;37:501–14.
-
Yamazaki M, Honda S, Tamaki K, Irie M, Mihara T. Effects of (+)-bicuculline, a GABAa receptor antagonist, on auditory steady state response in free-moving rats. PLoS ONE. 2020;15:e0236363.
-
Song TJ, Lan XY, Wei MP, Zhai FJ, Boeckers TM, Wang JN, et al. Altered behaviors and impaired synaptic function in a novel rat model with a complete Shank3 deletion. Front Cell Neurosci. 2019;13:111.
-
Jin QQ, Wu GQ, Peng WW, Xia XL, Hu L, Iannetti GD. Somatotopic representation of second pain in the primary somatosensory cortex of humans and rodents. J Neurosci. 2018;38:5538–50.
-
Hu L, Xia XL, Peng WW, Su WX, Luo F, Yuan H, et al. Was it a pain or a sound? across-species variability in sensory sensitivity. Pain. 2015;156:2449.
-
Xia XL, Peng WW, Iannetti GD, Hu L. Laser-evoked cortical responses in freely-moving rats reflect the activation of C-fibre afferent pathways. Neuroimage. 2016;128:209–17.
-
Lee MC, Mouraux A, Iannetti GD. Characterizing the cortical activity through which pain emerges from nociception. J Neurosci. 2009;29:7909–16.
-
Valentini E, Hu L, Chakrabarti B, Hu Y, Aglioti SM, Iannetti GD. The primary somatosensory cortex largely contributes to the early part of the cortical response elicited by nociceptive stimuli. Neuroimage. 2012;59:1571–81.
-
Ren W, Huang K, Li Y, Yang Q, Wang L, Guo K, et al. Altered pupil responses to social and non-social stimuli in Shank3 mutant dogs. Mol Psychiatry. 2023;28:3751–9.
-
Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. 2007;5:e133.
-
Peng W, Xia X, Yi M, Huang G, Zhang Z, Iannetti G, et al. Brain oscillations reflecting pain-related behavior in freely moving rats. Pain. 2018;159:106–18.
-
Zhang LB, Chen YX, Li ZJ, Geng XY, Zhao XY, Zhang FR, et al. Advances and challenges in neuroimaging-based pain biomarkers. Cell Rep Med. 2024;5:101784.
-
Schulz E, Tiemann L, Witkovsky V, Schmidt P, Ploner M. Gamma oscillations are involved in the sensorimotor transformation of pain. J Neurophysiol. 2012;108:1025–31.
-
Li Z, Zhang L, Zeng Y, Zhao Q, Hu L. Gamma-band oscillations of pain and nociception: a systematic review and meta-analysis of human and rodent studies. Neurosci Biobehav Rev. 2023;146:105062.
-
May ES, Nickel MM, Ta Dinh S, Tiemann L, Heitmann H, Voth I, et al. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum Brain Mapp. 2019;40:293–305.
-
Rojas DC, Wilson LB. γ-band abnormalities as markers of autism spectrum disorders. Biomark Med. 2014;8:353–68.
-
Simon DM, Wallace MT. Dysfunction of sensory oscillations in autism spectrum disorder. Neurosci Biobehav Rev. 2016;68:848–61.
-
Tierney AL, Gabard-Durnam L, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Developmental trajectories of resting EEG power: an endophenotype of autism spectrum disorder. PLoS ONE. 2012;7:e39127–e39127.
-
Buzsáki G, Logothetis N, Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–64.
-
Roche KJ, LeBlanc JJ, Levin AR, O’Leary HM, Baczewski LM, Nelson CA. Electroencephalographic spectral power as a marker of cortical function and disease severity in girls with Rett syndrome. J Neurodev Disord. 2019;11:15.
-
Wilkinson CL, Nelson CA. Increased aperiodic gamma power in young boys with fragile X syndrome is associated with better language ability. Mol Autism. 2021;12:17.
-
Molina JL, Voytek B, Thomas ML, Joshi YB, Bhakta SG, Talledo JA, et al. Memantine effects on electroencephalographic measures of putative excitatory/inhibitory balance in schizophrenia. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;5:562–8.
-
Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, et al. Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol Psychiatry. 2017;81:848–61.
-
Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27.
-
Zhu F, Shi Q, Jiang Y, Zhang YQ, Zhao H. Impaired synaptic function and hyperexcitability of the pyramidal neurons in the prefrontal cortex of autism-associated Shank3 mutant dogs. Mol Autism. 2024;15:9.
-
Jun HW. Pharmacokinetic studies of pentylenetetrazol in dogs. J Pharm Sci. 1976;65:1038–41.
-
Kirkpatrick DR, McEntire DM, Hambsch ZJ, Kerfeld MJ, Smith TA, Reisbig MD, et al. Therapeutic basis of clinical pain modulation. Clin Transl Sci. 2015;8:848–56.
-
Cetin FH, Tunca H, Güney E, Iseri E. Neurotransmitter systems in autism spectrum disorder. In: Fitzgerald M (ed). Autism Spectrum Disorder-Recent Advances. InTech: London. 2015, pp 15–30.
Acknowledgements
We thank Professor K. Guo and members of the Zhang laboratory for discussion. This work was supported in part by grants from the National Key Research and Development Program (2021ZD0203901 to Y.Z.), the National Science Foundation of China (32394030 to Y.Z., 32071061 to L.H.), the Beijing Natural Science Foundation (JQ22018 to L.H.), Wuhan Municipal S&T Project (Grant No. 2024020702030125 to Y.Z.), and sample storage by the Canine Biobank, Chinese Academy of Sciences (KFJ-BRP-004 to Y.Z.).
Author information
Authors and Affiliations
Contributions
Conceptualization: QS, LH, YZ; Methodology: QS, LH; Software: QS, LH; Validation: QS; Formal analysis: QS; Investigation: QS, BR, LW; Resources: LH, YZ; Data curation: QS; Writing – original draft preparation: QS, LH, YZ; Writing – review & editing: XL, LZ, YZ, LH; Visualisation: QS; Supervision: LH, YZ; Project administration: LH, YZ; Funding acquisition: LH, YZ.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Q., Ren, B., Lu, X. et al. Neural mechanisms underlying reduced nocifensive sensitivity in autism-associated Shank3 mutant dogs.
Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-02952-y
-
Received:
-
Revised:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41380-025-02952-y
This post was originally published on this site be sure to check out more of their content.