Abstract
Declining metabolic function with aging is a conserved phenotype across many species. While aging-associated changes in metabolic status have been investigated rigorously in humans, less is known about metabolic aging in dogs. In this cross-sectional study, we aimed to examine changes in metabolic health with age, and any associations with frailty and quality of life, in a diverse population of companion dogs. This cross-sectional study enrolled 451 mature, adult companion dogs. Serum adiponectin, ALP, ALT, AST, cholesterol, insulin, IGF-1 and glucose levels were quantified. Additionally, plasma FFA, SFA, PA, OA and LA were quantified in a 61 dog subpopulation. All analytes were significantly associated with age, with the exception of AST. Elevated ALP, ALT, cholesterol, insulin, FFA, PA and OA were correlated with increased frailty scores, while higher levels of glucose and adiponectin were correlated with reduced frailty scores. The strength of these associations increased with age. Higher ALP, ALT and insulin were associated with lower HRQL scores after adjusting for covariates. Our findings establish novel associations between deleterious aging-associated metabolic changes and validated measures of clinical well-being in companion dogs. Future research should investigate the causality of these associations to inform therapeutic strategies targeting age-associated changes to frailty and quality of life.
Introduction
Several core mechanisms of aging are highly conserved across species as diverse as worms, flies, rodents, and humans. For example, one common element of aging is metabolic dysfunction, which is characterized by insulin resistance and hyperinsulinemia and by dysregulated lipid metabolism1,2.
Fasting insulin concentrations and glucose levels are often used in humans as indicators of metabolic dysfunction and predictors of age-associated disease3,4. Both hyperinsulinemia and insulin resistance are well described characteristics of advanced aging and also risk factors for age-related diseases like sarcopenia, cardiovascular disease, and neurodegenerative disorders4.
In dogs, compensatory hyperinsulinemia does occur in response to peripheral insulin resistance5, and insulin levels increase with aging as in humans7,8,9,10. However, clear associations between chronic hyperinsulinemia and disease have not previously been reported in this species, though this may simply reflect the absence of data since insulin levels are rarely measured in dogs.
Another core element of aging-associated metabolic dysfunction across species is dysregulated lipid metabolism, adversely impacting triglyceride and cholesterol synthesis, lipolytic activity, and the rate of fatty acid oxidation. In humans, elevated fatty acid concentrations appear in states of metabolic dysregulation that occur with advancing age, including insulin resistance and hyperinsulinemia, and these elevations are associated with age-associated clinical conditions such as obesity and sarcopenia11,12,13,14.
Dogs also develop dyslipidemia associated with age and aging-related shifts in adipose distribution15,16,17,18,19,20. Some evidence suggests the lipidome may be associated with lifespan in dogs20, though again there is scant literature evaluating fatty acids and clinical outcomes in dogs.
In this cross-sectional study, we aimed to examine changes in metabolic health with age in a diverse population of client-owned companion dogs. This heterogeneous participant population encompasses dogs of varying age, size, breed and location, in an effort to describe changes in a real-world population as opposed to laboratory studies conducted in a homogeneous population. We measured multiple analytes, including ALP, AST, ALP, adiponectin, cholesterol, insulin, IGF-1 and glucose in serum samples previously collected from the participant population enrolled in a prior investigation21. Additionally, plasma FFA, SFA, PA, OA and LA were quantified by GC-MS in a subpopulation of 61 dogs representative of the total cohort. We then evaluated potential associations between these analytes and age, body weight, body condition and clinical measures of frailty and health-related quality of life.
We hypothesized that indicators of insulin resistance (e.g. fasting insulin and glucose) and dyslipidemia (e.g. cholesterol, triglycerides, and both individual and aggregated fatty acid levels), as well as adiponectin and other analytes previously identified as associated with negative health outcomes in aging (e.g. IGF-1), would show changes with age similar to those seen in humans.
We also expected that some of these indicators would be associated with clinical measures of frailty and quality of life. Frailty is a state of diminished function and physiologic resilience and an increased vulnerability to disability, disease, and death that occurs with aging. In humans, it is a commonly measured indicator that can predict important clinical outcomes such as loss of independence and mortality.
To assess frailty in our study population, we used the Canine Frailty Index (CFI) which has previously been shown to be predictive of short-term mortality risk and to correlate with quality of life as measured by another instrument, the VetMetrica Health-related Quality of Life tool (HRQL)21,22. Health-related quality of life reflects the negative impact of disease on overall well-being, and it commonly declines with aging.
Determining if patterns of metabolic dysfunction seen with aging in humans also occur in dogs, and identifying associations between these patterns and measures of important clinical outcomes, would be a significant contribution to our understanding of the mechanisms of aging and their potential clinical and translational impact.
Results
Descriptive statistics of biochemical analytes
Compared to young dogs in this cohort, old dogs were observed to have significantly higher levels of ALP (Young: 39.87 ± 56.02 U/L, Old: 168.17 ± 351.30 U/L, p < 0.001), ALT (Young: 41.17 ± 23.62 U/L, Old: 180.50 ± 191.71 U/L, p < 0.001), cholesterol (Young: 236.08 ± 63.17 mg/dL, Old: 252.21 ± 103.57 mg/dL, p = 0.041) and insulin (Young: 17.72 ± 12.49 mIU/L, Old: 20.72 ± 14.75 mIU/L, p = 0.028) compared to young dogs. Additionally, older dogs had significantly lower levels of adiponectin compared to young dogs (Young: 10,021.57 ± 6,005.98 ng/mL, Old: 5,521.02 ± 3,694.68 ng/mL, p < 0.001). No significant differences in AST, glucose, FFA, SFA, PA, OA and LA were observed between young and old dogs. Complete descriptive statistics for all serum and plasma biochemical analytes measured are reported in Table 1.
Biochemical analytes association with age
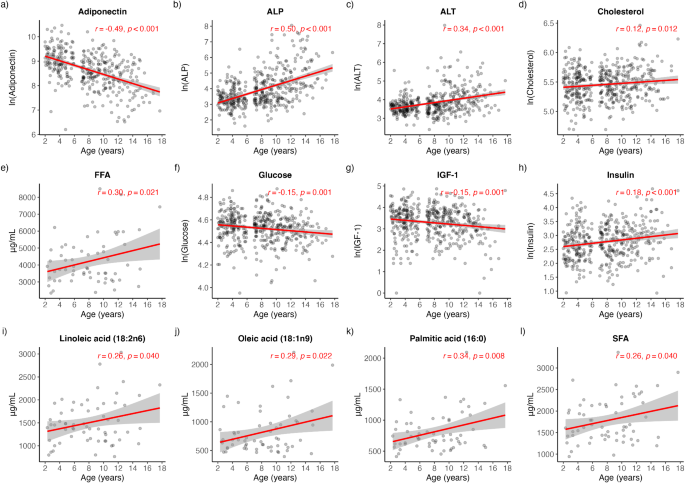
Pearson correlation analysis was performed on each biochemical analyte to determine its bivariate association with age. Significant positive associations with age were detected for ALP (rho = 0.50, p < 0.001), ALT (rho = 0.34, p < 0.001), cholesterol (rho = 0.12, p = 0.012), insulin (rho = 0.18, p < 0.001), FFA (rho = 0.30, p = 0.021), SFA (rho = 0.26, p = 0.040), PA (rho = 0.34, p = 0.006), OA (rho = 0.29, p = 0.022) and LA (rho = 0.26, p = 0.040) (Fig. 1b-e, h-l). Conversely, significant negative associations with age were detected for adiponectin (rho=-0.49, p < 0.001), glucose (rho=-0.15, p = 0.001) and IGF-1 (rho=-0.15, p = 0.001) (Fig. 1a, f-g). A multiple regression analysis was conducted to estimate the covariate-adjusted (weight, BCS, sex, gonadectomy status) associations with age. Adjusting for covariates, all analytes continued to significantly associate with age with the exception of IGF-1 (p = 0.839) (Table 2). As larger body size is highly correlated with IGF-1, a sensitivity analysis was performed where weight was removed as a covariate. In a model excluding weight as a covariate, IGF-1 was significantly negatively associated with age (Coefficient (95% CI) = -0.44 (-0.86, -0.01), p = 0.043) (Table 2).
Pearson’s correlations demonstrating the bivariate associations of serum and plasma biochemical analytes with age. (a) adiponectin (b) ALP (c) ALT (d) cholesterol (e) FFA (f) glucose (g) IGF-1 (h) insulin (i) linoleic acid (j) oleic acid (k) palmitic acid (l) SFA. ALP alkaline phosphatase, ALT alanine aminotransferase, FFA free fatty acids, IGF-1 insulin-like growth factor 1, SFA saturated fatty acids.
Biochemical analytes association with CFI
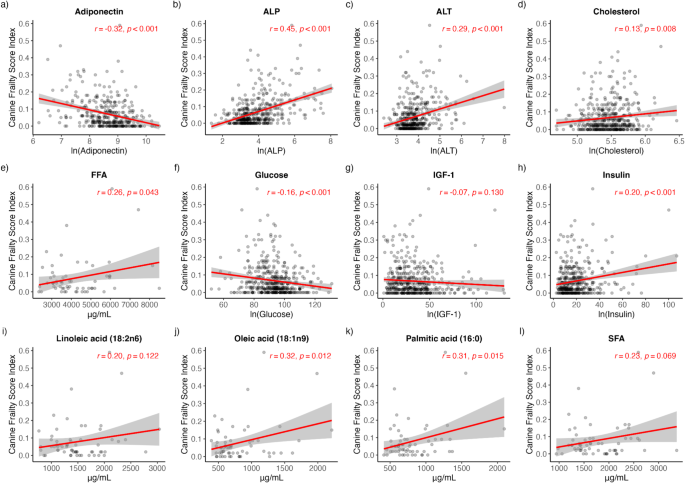
Pearson correlation analysis was performed on each biochemical analyte to determine its bivariate association with CFI. Significant positive associations with CFI were detected for ALP (rho = 0.45, p < 0.001), ALT (rho = 0.29, p < 0.001), cholesterol (rho = 0.13, p = 0.008), FFA (rho = 0.26, p = 0.043), insulin (rho = 0.20, p < 0.001), OA (rho = 0.32, p = 0.012) and PA (rho = 0.31, p = 0.015) (Fig. 2b-e, h, j, k). Significant negative associations with CFI were detected for adiponectin (rho=-0.32, p < 0.001) and glucose (rho=-0.16, p < 0.001) (Fig. 2a, f). No significant associations with CFI were detected for IGF-1, SFA and LA (Fig. 2g, i, l).
Pearson’s correlations demonstrating the bivariate associations of serum and plasma biochemical analytes with CFI. (a) adiponectin (b) ALP (c) ALT (d) cholesterol (e) FFA (f) glucose (g) IGF-1 (h) insulin (i) linoleic acid (j) oleic acid (k) palmitic acid (l) SFA. ALP alkaline phosphatase, ALT alanine aminotransferase, FFA free fatty acids, IGF-1 insulin-like growth factor 1, SFA saturated fatty acids.
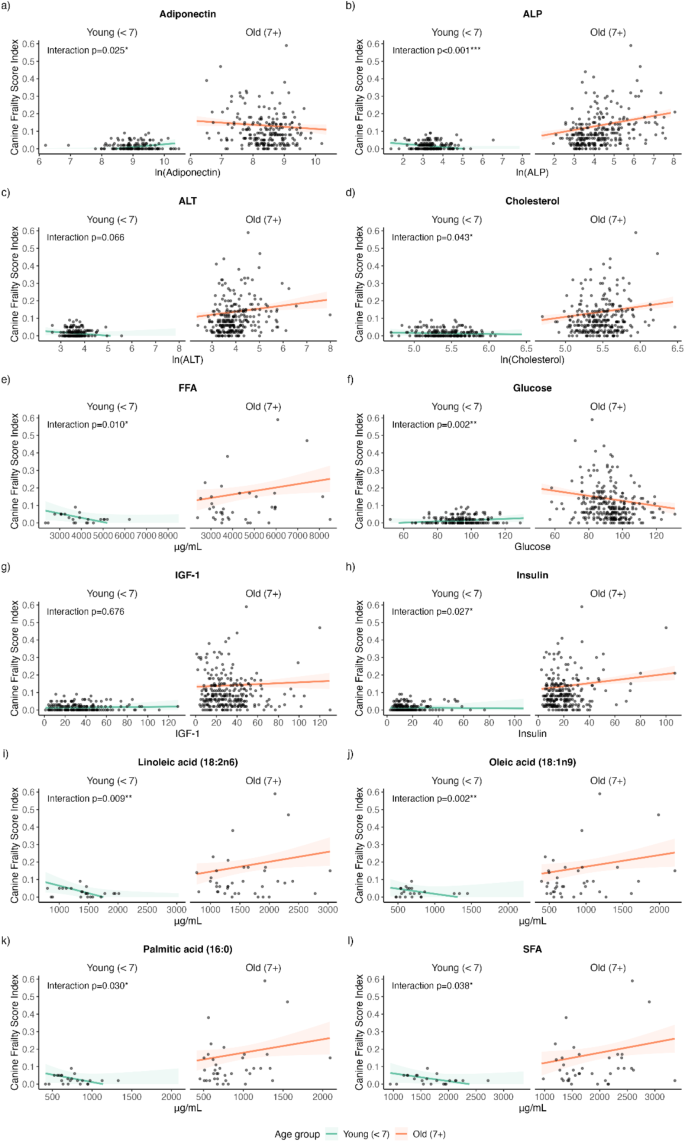
A multiple regression analysis was conducted to estimate the covariate-adjusted associations between biochemical analytes with CFI (main effect model) and their potential interaction with age (interaction model). After adjusting for covariates, only ALP (Coefficient (95% CI) = 0.14 (0.05, 0.22), p = 0.002) and insulin (Coefficient (95% CI) = 0.09 (0.01, 0.18), p = 0.031) were significantly positively associated with CFI when tested as a main effect (Table 3). However, these null findings can be explained by the presence of interaction effects with age. All biochemical analytes, except for IGF-1 (p = 0.676) showed significant interaction effects with age, providing evidence that these relationships strengthen with age (Table 3). Figure 3 provides visualization of how these associations change with age.
Interaction effects, presented as estimated marginal means, of serum and plasma biochemical analytes and age in their association with CFI. (a) adiponectin (b) ALP (c) ALT (d) cholesterol (e) FFA (f) glucose (g) IGF-1 (h) insulin (i) linoleic acid (j) oleic acid (k) palmitic acid (l) SFA. ALP alkaline phosphatase, ALT alanine aminotransferase, FFA free fatty acids, IGF-1 insulin-like growth factor 1, SFA saturated fatty acids.
Biochemical analytes association with HRQL
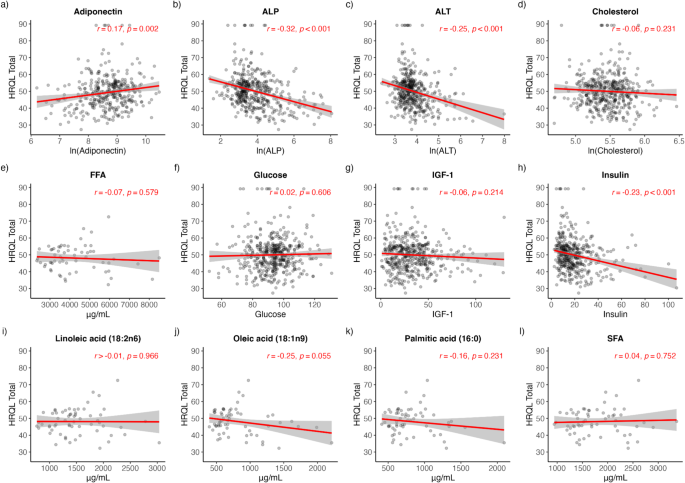
Pearson correlation analysis was performed on each biochemical analyte to determine its bivariate association with HRQL. A significant positive correlation with HRQL was detected only for adiponectin (rho = 0.17, p = 0.002), while significant negative correlations were detected for ALP (rho=-0.31, p < 0.001), ALT (rho=-0.25, p < 0.001) and insulin (rho=-0.23, p < 0.001) (Fig. 4a-c, h). No associations were detected for cholesterol, glucose, FFA, SFA, PA, OA and LA (Fig. 4d-g, i-l).
As with CFI, a multiple regression analysis was conducted to estimate the covariate-adjusted associations between biochemical analytes with HRQL (main effect model) and their potential interaction with age (interaction model). After adjusting for covariates, only ALP (Coefficient (95% CI) = -0.13 (-0.22, -0.30), p = 0.012), ALT (Coefficient (95% CI) = -0.12 (-0.21, -0.03), p = 0.007) and insulin (Coefficient (95% CI) = -0.15, (-0.24, -0.06), p = 0.002) maintained significant negative main effect associations with HRQL (Table 4), indicating that for dogs of the same age, increases in ALP, ALT, and insulin are associated with reductions in HRQL scores. Age did not significantly interact with any of these associations (Table 4).
Discussion
Several core elements of aging-associated metabolic dysfunction are well-characterized in humans and rodents, including development of peripheral insulin resistance and hyperinsulinemia, dyslipidemia, shifts in the amount and proportion of various circulating fatty acids, and changes in the distribution and function of adipose tissue23,24,25. Certain proteins, such as IGF-1 and adiponectin, and routine clinical chemistry analytes, such as ALT, AST, and ALP, have also been associated with metabolic dysfunction and with aging and aging-associated diseases in these species26,34,35,36,30.
While many of these changes are well-characterized in humans and rodents, few studies have evaluated alterations of metabolic function with aging in companion dogs31,32. Some investigations have shown changes similar to those seen in aging humans, such as hyperinsulinemia, hypoadiponectinemia, and some components of dyslipidemia7,31. However, not all studies have consistently reported changes in these parameters with aging, and they have not yet been clearly related to clinically significant outcomes, such as lifespan, quality of life, frailty, and diseases in geriatric dogs10,18,31,33,34,35,36.
The metabolic action and downstream signaling effects of insulin is one of the most exhaustively described processes in aging due the association with lifespan in multiple species6. Increases in fasting insulin are typically indicative of peripheral insulin resistance, which causes chronic hyperinsulinemic compensation by the pancreas in an effort to maintain glucose homeostasis. Some previous cross-sectional and longitudinal investigations conducted in young and old dogs have demonstrated increases in fasting insulin and in both tissue-specific and systemic insulin resistance, as well as reductions in insulin-stimulated GLUT4 expression and AKT phosphorylation8,9.
In humans and rodents, the presence of persistent hyperinsulinemia and insulin resistance is associated with higher rates of chronic diseases, such as type 2 diabetes, cognitive decline, sarcopenia, and cancer, leading to reduced quality of life and lifespan4,6,37,38. Our data appear to be the first to show that increases in fasting insulin are associated with poorer quality of life and increased frailty in aged dogs.
Treatment of hyperinsulinemia and insulin resistance is a common approach to preventing the progression of metabolic diseases in humans. Interventional studies across species have demonstrated that lowering fasting insulin and promoting insulin sensitivity can delay the onset of aging-associated disease and extend lifespan6. In dogs, for example, the preservation of insulin sensitivity with caloric restriction was independently associated with increased lifespan9.
Despite this, insulin is not commonly measured in canine veterinary patients unless there is a clinical suspicion for type I diabetes mellitus or an insulin-producing tumor. While further research is needed to demonstrate a causal relationship between insulin levels and lifespan, the significant associations in these data between insulin, frailty and quality of life support the hypothesis that hyperinsulinemia is a factor in the negative health outcomes resulting from metabolic dysfunction and aging. If this relationship is borne out, insulin may turn out to be a valuable clinical biomarker for assessing aging and metabolic health in dogs.
In humans and rodents, glucose concentrations are another common and important indicator of metabolic status. As metabolic function deteriorates with age in these species, glucose homeostasis is compromised, leading to reduced insulin-stimulated glucose disposal and increased fasting blood glucose levels.
Previous evidence, though limited, has indicated that glucose levels remain unchanged or decline with aging in dogs10,18,31,33,34,36. Our data also show that fasting glucose decreases modestly with age after adjusting for covariates. This decrease was associated with an increase in frailty, and this relationship became stronger with age.
These findings differ from the typical pattern seen in humans and rodents. Insulin levels increased with age in the study dogs, presumably reflecting the same increase in peripheral insulin resistance commonly seen with aging in other species. However, glucose levels declined rather than increased despite this.
The regulation and significance of plasma glucose levels in dogs appear to differ significantly from that in humans and rodents. Diabetes mellitus is far less prevalent in dogs than humans, and it is almost always due to a primary deficiency in insulin production rather than the development of peripheral insulin resistance39,40. And while diabetes can be diagnosed in humans by identifying persistent fasting hyperglycemia, the presence and severity of hyperglycemia in dogs, when it does occur, does not appear consistently associated with an eventual progression to insulin-dependent diabetes41.
Even in the face of robust metabolic stressors, such as a high-fat diet, dogs maintain the ability to increase insulin secretion in order to maintain euglycemia42,48. Investigations into overweight and obese dogs have shown only modest elevations in fasting glucose (~ 3-6 mg/dL) compared to dogs with a normal body condition43. Plasma glucose levels, therefore, appear unlikely to be an informative indicator of metabolic dysfunction associated with aging or other causes in this species.
Dyslipidemia and dysregulated lipid metabolism is another element of aging-associated metabolic dysfunction which has been characterized across multiple species44,45. Our data confirm the findings of some previous studies showing increases in total cholesterol with age in dogs31,34. We also observed that certain circulating plasma fatty acids associated with metabolic dysfunction in humans are also elevated with age in dogs, which is a novel finding. Furthermore, this study is the first to identify that elevated levels of cholesterol and fatty acids in circulation are associated with increased frailty. This association gets stronger as dogs age, suggesting that the deleterious consequences of dyslipidemia appear to increase with increasing age in dogs as they do in humans.
Dyslipidemia and dysregulated lipid metabolism in humans are associated with the expansion and dysfunction of the adipose tissue, and these are considered modifiable risk factors for multiple metabolic and cardiovascular diseases, such as coronary artery disease (CAD)25,46. Dogs do not develop CAD, likely due to the relative abundance of HDL in comparison to LDL in dogs47, and modest changes in blood lipids with aging are not typically seen as cause for concern or intervention in this species.
However, in previous studies we have shown that high levels of triglycerides and cholesterol are associated with elevated fasting insulin and hyperinsulinemic compensation in dogs fed both standard and high fat diets42,48. Given the association between hyperinsulinemia and disease in humans and other species, and the novel finding in this study that elevated fasting insulin is associated with frailty and declining quality of life, further investigation into the clinical significance and predictive value of blood lipids in aging dogs is warranted.
Another aspect of dysregulated lipid metabolism is the inability of adipose tissue to effectively respond to insulin by shutting down lipolysis, leading to fatty acid spillover in the circulation. Across rodents, humans and dogs, higher levels of plasma fatty acids (e.g. FFA, SFA, PA, OA, LA) in circulation are associated with decreased insulin sensitivity, as shown by glucose tolerance tests and hyperinsulinemic euglycemic clamp42,48,49,50,51. Laboratory studies have also demonstrated that the elevation of circulating triglycerides and fatty acids through lipid infusions increases peripheral insulin resistance52.
In this study, circulating fatty acids were positively associated with age. Increasing fatty acid levels were also independently associated with frailty, and this relationship strengthened with increasing age. These associations, and the similar relationships discussed above between hyperinsulinemia, age, and frailty, are suggestive of ongoing insulin resistance and adipose dysfunction in aged dogs. Notably, ω-3 fatty acid concentrations, which are generally considered beneficial, were not associated with age, CFI or HQRL (Supplemental Table 3). Additional research is needed to fully elucidate the impact of dyslipidemia and dysregulated lipid metabolism on frailty in both dogs and humans.
Adiponectin has been extensively studied in the context of aging due to observed high levels in extremely long-lived humans and the association of low adiponectin levels with the development of age-associated diseases53,54. The pleiotropic effects of adiponectin include activating AMPK and PPARα while blocking downstream effects NF-κB, thereby increasing insulin action and fatty acid oxidation and reducing inflammatory tone27,55. Our data demonstrate adiponectin levels are diminished in aged dogs, as they typically are in aging humans. Lower adiponectin levels are also associated with lower quality of life and increased frailty in dogs, though the association with quality of life did not persist after adjusting for relevant covariates.
Low levels of adiponectin are associated with multiple cardiometabolic risk factors and diseases in humans, including obesity, increased visceral adiposity and inflammation56. Preclinical studies in rodents have demonstrated that genetic or pharmacologic interventions to increase adiponectin can reduce inflammation and improve insulin sensitivity and dyslipidemia in models of metabolic dysfunction (e.g. high fat diet) and in aging animals57,58. Currently, there has been little investigation into the association between adiponectin and disease in dogs, with only a few studies linking low adiponectin to aging-associated disease59,60,61. More research is needed to determine if adiponectin is integral to metabolic health and the manifestation of aging and frailty in dogs.
AST, ALP and ALP are routinely measured enzymes in both human and veterinary medicine. Clinically, they are used as indicators of hepatic injury, though they are not always specific markers of primary hepatobiliary disease62,63. Increases in AST and ALT are biochemical markers of hepatocyte damage, though AST is less specific and also associated with bone while ALT can also be elevated by hemolysis and muscle damage. Elevated ALP typically indicates cholestasis, though there are various isozymes that can be associated with bone metabolism or induced by glucocorticoids or other factors62,63.
In humans, elevations ALT and ALP are closely monitored as they are associated with fatty liver, insulin resistance and mortality28,64,65. However, in dogs elevations of these enzymes are common, and determining their clinical significance can be complex and must include consideration of the total context of each specific case34.
Our data reveal that both ALT and ALP increased with age in the dogs in this cohort, while no such association was seen with AST. Increases in both ALP and ALT were also associated with increased frailty and decreased quality of life. Interestingly, the absolute values of these analytes were always within the reference ranges, suggesting that clinically meaningful changes may occur within those limits. Elevations in ALT and ALP are often not considered clinically significant unless they are moderate to severe (often greater than 4–5 fold the upper end of the reference range) or persistent over several weeks66. However, these guidelines may only be applicable for identifying acute or extensive hepatic dysfunction, and our results suggest that more subtle changes may be useful early indicators of meaningful metabolic dysfunction.
These data replicate similar findings in humans that ALP levels are increased with age and associated with higher levels of cardiovascular risk and all cause mortality even when remaining largely within reference range66. More investigation is needed into age-associated increases of these enzymes and their clinical relevance in dogs.
Another analyte that has a well-established role in aging is IGF-1. Elevated IGF-1 levels have been associated with reduced lifespan in humans, rodents, and dogs, and interventions that lower IGF-1 have been shown to extend lifespan in multiple species, including worms, flies, mice, and humans67. The specific effects of IGF-1 that influence longevity are complex and incompletely characterized. However, it has been demonstrated that interventions which prolong lifespan by improving metabolic health, such as caloric restriction, may also suppress IGF-168. It appears that lower IGF-1 may be associated with improved insulin sensitivity, increased adiponectin, and preservation of metabolic health during aging69.
In dogs, IGF-1 levels vary dramatically between dogs of different breeds due to the effects of intense artificial selection for body size. Large and giant-breed dogs have much higher lifelong levels of IGF-1, and this is associated with shorter lifespan and with frailty70,71,72. Based on these and other unpublished data, we expected to see an association between IGF-1 and age, biomarkers of metabolic dysfunction, and measures of frailty and quality of life.
IGF-1 was negatively associated with age, however this association did not remain after adjusting for several covariates, including weight. IGF-1 was also not significantly associated with frailty or quality of life in adjusted regression analyses that included weight as a covariate, though it was inversely associated with quality of life when weight was removed from the model (data not shown). These results suggest that the extremely strong relationship between body size and IGF-1 levels overshadows any other statistical associations between IGF-1 and indicators of age-related metabolic health and frailty or quality of life. The existing evidence still strongly suggests that IGF-1 plays an important role in lifespan and healthspan through its effects on metabolic dysfunction.
In this study, we found that a diverse cohort of companion dogs manifested several aging-associated changes in metabolic health previously recognized in humans and other species. These include increases in insulin, circulating fatty acids, ALT and ALP, and a decrease in adiponectin. We also found patterns in dogs different from those reported in species such as rodents and humans, including a decrease in plasma glucose with age despite hyperinsulinemia and apparent increasing insulin resistance.
These data demonstrate an association between these deleterious metabolic changes and validated measures of clinical well-being, including frailty and health-related quality of life. Future research should determine which, if any, of these relationships are causal and how further understanding of them may impact predicting and preserving healthspan and lifespan in dogs.
Limitations of study
Limitations of this study include that measurements were assessed after a minimum of 6 h of fasting, and fasting was determined by attestation from the dog owners and not independently verified. Although the VetMetrica HRQL and CFI methods are established methods to assess HRQL and frailty, subjectivity and bias from pet owners completing the HRQL questionnaire and interobserver variability in clinicians scoring for the CFI assessment could have affected HRQL and CFI scores, respectively.
The cohort evaluated was a convenience sample selected to meet predetermined criteria of age and size. It is possible that this group may not be representative of all companion dogs in terms of breed, neuter status, and other relevant variables. There were also some differences between the young and old subgroups, with larger and intact dogs being more common in the young subgroup, though adjustment for BCS and neuter status during regression analysis should account for these differences. Plasma fatty acid quantification was only conducted in a subset (n = 61) of dogs with the remaining sample from the full study cohort (n = 451).
Finally, the cross-sectional nature of this study only provides a single timepoint to examine the differences in clinical and physiological status of dogs at various ages. A longitudinal study following the same dogs across multiple sampled timepoints as they age would be required to investigate potentially dynamic changes in physiology and clinical status with aging.
Methods
Cohort characteristics
Demographics of the dog cohort enrolled in this study were previously described by Chen et al.21 Briefly, 451 adult companion dogs were enrolled. The cohort sampled was made up of 182 (40.35%) young dogs (< 7 years) and 269 (59.65%) old dogs (7 + years), 46.3% female dogs and 53.7% male dogs, 91.1% of which were neutered. The average age and weight of dogs sampled was 7.72 ± 3.76 years and 24.01 ± 15.37 kg. The evaluable population consisted of 43.6% mixed breeds and 56.4% purebred dogs. Old dogs in this cohort were reported to have significantly lower body weights and higher rates of gonadectomy (p = 0.011 and p < 0.001, respectively). Age cutoffs were determined based on previous research norms to facilitate comparison between studies21,22. Similarly, consistent with previous research studies21,22, weight cutoffs were selected based on AKC dog breed classification for small- and large-sized dogs73. A complete demographic profile of this cohort is reported in Table 5 and list of the most common breeds in this cohort is reported in Supplement Table 2.
Additionally, a subset of 61 dogs had their plasma fatty acid profiles quantified by targeted lipidomics at Metabolon Inc (Morrisville, NC). On average, animals were 8.2 years old, 25.5 kg and 52.5% female. Demographic information and descriptive statistics for age, sex, desexed status, purebred (yes/no), weight, BCS, HRQL total scores, HRQL domain scores, CFI scores, insulin (mU/L) concentrations and fatty acid concentrations (µg/mL) for the 61 dog subset analyzed in Supplement Table 1.
This study was performed according to VICH-GL9 Good Clinical Practice (GCP) standards. Approval by an independent IACUC was obtained on 13 Dec 2020 through Veterinary and Biomedical Research Center, Inc. (VBRC) (Approval no.: VACLOY001CLDEFF-1PILC). All investigators and study veterinarians were qualified, licensed veterinarians. Owner informed consent was obtained prior to any study procedures. All dogs were determined to be in good health with no clinically significant abnormalities on physical examination or clinical laboratory analysis. This study is reported in accordance with ARRIVE guidelines.
Sample collection
Fasted blood samples were collected via venipuncture in a serum separator tube. The protocol required at least 6 h of fasting, although this was not confirmed at the time of collection. Samples were left to clot at room temperature for no more than 60 min prior to centrifugation at 2800–3200 rpm for 10 min at ambient temperature. From each sample, the resultant serum was divided into aliquots of approximately 500–1000µL using 1.8 mL cryovials and stored at approximately − 80 °C.
VetMetrica HRQL scores
Scores were available for four VetMetrica HRQL sub-domains: Energetic/Enthusiastic (E/E), Happy/Content (H/C), Active/Comfortable (A/C), and Calm/Relaxed (C/R). Individual HRQL domain scores range from 0 to 6, with larger scores indicating higher quality of life in that domain. Following Davies et al., HRQL total score was calculated as the sum of all four domains, which was then standardized and rescaled such that total scores ranged from 0 to 100 with a mean of 50 and a standard deviation of 10. The HRQL total score represents a dog’s overall quality of life, where higher scores indicate better quality of life and lower scores lower quality of life.
CFI scores
The CFI was developed as a tool to measure frailty through the assessment of multimorbidity and functional deficits associated with aging in dogs47. The CFI was developed as an analog to the frailty index used in humans or rodents, where frailty is assessed across multiple dimensions and reflects a general state of poor health. The CFI contains 33 discrete clinical health items, ranging from yes/no answers on functional ability (e.g., “Assistance for standing up”, with responses as “Yes” = 1 or “No” = 0) to specific disease states (e.g. “Disease of the oral category”, with responses as “No” = 0, “Mild” = 0.5, “Severe” = 1). These items were assessed by a veterinarian. Scores were then totaled (maximum value of 33) and then divided by the total number of items, such that CFI scores range from 0 to 1, with 1 indicating the highest frailty.
Serum biochemistry
The following serum biochemistry tests were performed and analyzed by IDEXX BioAnalytics (West Sacramento, CA): Albumin/Globulin (ALB/GLOB) ratio, Albumin, Alkaline Phosphatase (ALP), Alanine Transferase (ALT), Aspartate transaminase (AST), Bicarbonate, Bilirubin – Conjugated, Bilirubin – Unconjugated, Blood Urea Nitrogen (BUN), BUN/Creatinine Ratio, Calcium, Chloride, Cholesterol, Creatine kinase, Creatinine, Globulin, Glucose, Hemolysis Index, Lipemia Index, Na/K Ratio, Phosphorus, Potassium, Sodium, Total Bilirubin, Total Protein.
Adiponectin quantification
Serum samples were analyzed for adiponectin concentrations by using a commercially available sandwich enzyme-linked immunoassay (ELISA) (Circulex Dog Adiponectin ELISA kit, MBL International; CY-8052) at Eve Technologies (Calgary, Canada) as per the kit manufacturer’s instructions. A precoated microplate with an immobilized antibody for adiponectin was loaded with serum samples (diluted 1:5000) or concentration standards for binding. After washing away unbound substances, a horseradish peroxidase (HRP) conjugated antibody for adiponectin was added to the wells. Incubation was performed for 60 min at 25 °C on a plate shaker (300 rpm). The excess unbound enzyme-labeled antibody was washed again and the remaining conjugate was allowed to react with an H2O2-tetramethylbenzidine solution. The assay was terminated by adding an acidic solution and read at 450 nm wavelength on a microplate reader. A standard curve was generated by plotting absorbance values versus known adiponectin concentrations of calibrators, and concentrations of the unknown serum samples were determined using that curve.
IGF-1 quantification
Serum samples were analyzed for IGF-1 concentrations by radioimmunoassay at Michigan State University, Veterinary Diagnostic Laboratory (MSU VDL, laboratory of Dr. Brian Petroff PhD, DVM) using the protocol previously described (Hofer-Inteeworn et al., 2012). IGF-1 was measured in prediluted samples (1:100) in an acidification buffer to achieve dissociation of IGF-1 from its binding proteins. An aliquot of the acidified sample is mixed with a de-acidifying reagent containing an anti-IGF1 antibody and concentrated amount of IGF-1. Radiolabeled IGF-1 was added to the assay to compete with the endogenous IGF-1 for binding to the antibody. After incubation, the antibody-bound radioligand is isolated with a second antibody, precipitating agent, followed by centrifugation. Linearity was assessed by comparing results from 3 serum samples prepared at various dilutions (1:1. 1:2 and 1:3) with acidification and buffer.
Insulin quantification
Serum samples were analyzed for insulin concentrations using a commercially available sandwich ELISA (10-1203-01) by Mercodia (Sweden) per the kit manufacturer’s instructions. A precoated microplate with an immobilized antibody for insulin was loaded with serum samples or concentration standards for binding. After washing away unbound substances, an HRP conjugated antibody for insulin was added to the wells. Incubation was performed for 2 h at 18–25 °C on a plate shaker (700-900 rpm). The excess unbound enzyme-labeled antibody was washed again and the remaining conjugate was allowed to react with a 3,3 ́-5,5 ́-tetramethylbenzidine solution. The assay was terminated by adding an acidic solution and read at 450 nm wavelength on a microplate reader. A curve was generated from the known concentration standards and the unknown serum samples were determined using that curve.
Fatty acid quantification
Plasma samples were analyzed by gas chromatography-mass spectrometry (GC/MS; Metabolon, Inc) for the determination of the total content of FFAs (palmitic acid, palmitoleic acid, margaric acid, stearic acid, oleic acid, linoleic acid, gamma linoleic acid, alpha-linoleic acid); SFAs (myristic acid, pentadecanoic acid, palmitic acid, stearic acid, arachidic acid). Behenic, nervonic and lignoceric acids were also quantified, however they did not pass assay QC so their data is not included. Aliquots of plasma were pipetted into tubes and lyophilized. Internal standard solution was added to the lyophilized plasma samples. The solvent was removed by evaporation under a stream of nitrogen. The dried sample was subjected to methylation/transmethylation with methanol/sulfuric acid, resulting in the formation of the corresponding methyl esters of free fatty acids and conjugated fatty acids. The reaction mixture was neutralized and extracted with hexanes. An aliquot of the hexanes layer was injected onto a 7890 A/5975 C GC/MS system (Agilent Technologies, CA). Mass spectrometric analysis was performed in the single ion monitoring positive mode with electron ionization. Quantitation was performed using both linear and quadratic regression analysis generated from fortified calibration standards prepared immediately prior to each run. Raw data was collected and processed using Agilent MassHunter GC/MS Acquisition B.07.04.2260 and Agilent MassHunter Workstation Software Quantitative Analysis for GC/MS B.09.00/ Build 9.0.647.0 (Agilent Technologies, CA). Data reduction was performed using Microsoft Office 365 ProPlus Excel (Microsoft, WA).
Statistical analyses
Descriptive statistics
Descriptive statistics (N, mean, standard deviation, median, 25th and 75th percentiles, minimum and maximum) of demographic features (age, sex, desexed status, % pure or mixed breed), biochemical analytes and clinical outcome variables (HRQL total and CFI scores) were summarized for the entire dataset as well as stratified by old (≥ 7 years of age) and young (≥ 2 and < 6 years of age). Two-sample t-tests and chi-square tests were used, where appropriate, to compare differences in these variables across old and young dogs.
Fatty acid sub-population
To confirm that the sub-population of dogs within the Healthspan cohort which had fatty acid quantification (n = 61) did not differ substantially from the full cohort, (n = 451), descriptive statistics (N, mean, standard deviation, median, 25th and 75th percentiles, minimum and maximum) of demographic features (age, sex, desexed status, % pure or mixed breed) and clinical outcome variables (HRQL total and CFI scores) were reported for these dogs, as well as stratified by old (≥ 7 years of age) and young (≥ 2 and < 6 years of age). Histograms comparing key demographic variables across the fatty acid sub-population and the full cohort were also presented and reported in the supplemental material.
Bivariate correlations
Pearson’s correlation was used to estimate the bivariate relationships between individual analyte and (1) age, (2) HRQL Total scores and, (3) CFI scores. Violations of non-normality and/or non-linearity assumptions were addressed using natural log-transformations where appropriate. Results of correlation analyses were reported in scatterplots or tables with correlation coefficients and corresponding p-values reported.
Multiple regression
Multiple linear regression was used to test covariate adjusted main effect relationships between each analyte and the following outcomes (1) age, (2) HRQL Total scores, and (3) CFI scores. Body weight (kg), Body Condition Score (BCS), neuter status (Desexed or Intact) and sex (Male or Female) were included as covariates for all models. As relationships between biochemical analytes and clinical outcomes (HRQL and CFI) might change with age, interactions between each blood-based biomarker and age (both continuous) were tested. If any interaction effects were detected, visualization of these effects were provided using estimated marginal predictions assuming mean values across all continuous covariates.
Issues of non-normality and/or non-linearity were addressed using natural log-transformations where appropriate. Issues of heteroskedasticity were addressed using the Huber robust sandwich estimator to calculate standard errors and to perform inference. To facilitate interpretation of regression coefficients across outcomes and predictors with vastly different scales, standardization (mean = 0, standard deviation = 1) was used where appropriate. Tables of multiple regression analyses report covariate-adjusted coefficients and their 95% confidence intervals, as well as any transformations performed or standard errors used.
All analyses and data presentations were performed using R version 4.3.2.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
References
-
Rochlani, Y., Pothineni, N. V., Kovelamudi, S. & Mehta, J. L. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 11, 215–225 (2017).
-
Fahed, G. et al. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int J Mol Sci. 23, (2022).
-
Facchini, F. S., Hua, N., Abbasi, F. & Reaven, G. M. Insulin resistance as a predictor of age-related diseases. J. Clin. Endocrinol. Metab. 86, 3574–3578 (2001).
-
Kolb, H., Kempf, K. & Martin, S. Insulin and aging – a disappointing relationship. Front. Endocrinol. (Lausanne). 14, 1261298 (2023).
-
Ader, M. & Bergman, R. N. Hyperinsulinemic Compensation for Insulin Resistance Occurs Independent of Elevated Glycemia in male dogs. Endocrinology 162, 1–9 (2021).
-
Janssen, J. A. M. J. Hyperinsulinemia and its pivotal role in aging, obesity, type 2 diabetes, Cardiovascular Disease and Cancer. Int. J. Mol. Sci. 22, 7797–7822 (2021).
-
Lawler, D. F. et al. Diet restriction and ageing in the dog: major observations over two decades. Br. J. Nutr. 99, 793–805 (2008).
-
Bhashyam, S. et al. Aging is associated with myocardial insulin resistance and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 293, H3063–3071 (2007).
-
Larson, B. T., Lawler, D. F., Spitznagel, E. L. & Kealy, R. D. Improved glucose tolerance with lifetime diet restriction favorably affects disease and survival in dogs. J. Nutr. 133, 2887–2892 (2003).
-
Strasser, A., Niedermüller, H., Hofecker, G. & Laber, G. The effect of aging on laboratory values in dogs. Zentralbl Veterinarmed A. 40, 720–730 (1993).
-
Al Saedi, A., Debruin, D. A., Hayes, A. & Hamrick, M. Lipid metabolism in Sarcopenia. Bone 164, 116539 (2022).
-
Boden, G. & Chen, X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J. Clin. Invest. 96, 1261–1268 (1995).
-
Walle, P. et al. Pihlajamäki. Fatty acid metabolism is altered in non-alcoholic steatohepatitis independent of obesity. Metabolism 65, 655–666 (2016).
-
Mesinovic, J., Zengin, A., De Courten, B., Ebeling, P. R. & Scott, D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab. Syndr. Obes. 12, 1057–1072 (2019).
-
Lottati, M., Kolka, C. M., Stefanovski, D., Kirkman, E. L. & Bergman, R. N. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obes. (Silver Spring). 17, 674–680 (2009).
-
German, A. J. et al. Trayhurn. Improvement in insulin resistance and reduction in plasma inflammatory adipokines after weight loss in obese dogs. Domest. Anim. Endocrinol. 37, 214–226 (2009).
-
Müller, L. et al. Thuróczy. Body fat distribution and metabolic consequences – examination opportunities in dogs. Acta Vet. Hung. 62, 169–179 (2014).
-
Kawasumi, K. et al. Age effects on plasma cholesterol and triglyceride profiles and metabolite concentrations in dogs. BMC Vet. Res. 10, 57 (2014).
-
Turner, R. B. S., Tyrrell, D., Hepworth, G., Dunshea, F. R. & Mansfield, C. S. Compartmental fat distribution in the abdomen of dogs relative to overall body fat composition. BMC Vet. Res. 16, 104 (2020).
-
Hoffman, J. M., Kiklevich, J. V., Klavins, K., Valencak, T. G. & Austad, S. N. Alterations of lipid metabolism with Age and Weight in Companion Dogs. J. Gerontol. Biol. Sci. Med. Sci. 76, 400–405 (2021).
-
Chen, F. L. et al. LaCroix-Fralish. Evaluating instruments for assessing healthspan: a multi-center cross-sectional study on health-related quality of life (HRQL) and frailty in the companion dog. Geroscience 45, 2089–2108 (2023).
-
Banzato, T. et al. Canevelli. A. A Frailty Index based on clinical data to quantify mortality risk in dogs. Sci. Rep. 9, 16749 (2019).
-
Marmentini, C. et al. Kurauti. Aging reduces insulin clearance in mice. Front. Endocrinol. (Lausanne). 12, 679492 (2021).
-
Chang, A. M. & Halter, J. B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 284, E7–12 (2003).
-
Huffman, D. M. & Barzilai, N. Role of visceral adipose tissue in aging. Biochim. Biophys. Acta. 1790, 1117–1123 (2009).
-
Junnila, R. K., List, E. O., Berryman, D. E., Murrey, J. W. & Kopchick, J. J. The GH/IGF-1 axis in ageing and longevity. Nat. Rev. Endocrinol. 9, 366–376 (2013).
-
Iwabu, M., Okada-Iwabu, M., Yamauchi, T. & Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 1, 15013 (2015).
-
Liu, C. et al. Obesity, insulin resistance and their interaction on liver enzymes. PLoS One. 16, e0249299 (2021).
-
Yang, F. et al. Predicting life span of type 2 diabetes patients through alkaline phosphatase and vitamin D: results from NHANES 1999–2018. Atherosclerosis 394, 117318 (2024).
-
Nolasco, E. L. et al. Martins. Insulin modulates liver function in a type I diabetes rat model. Cell. Physiol. Biochem. 36, 1467–1479 (2015).
-
Mori, N., Kawasumi, K. & Arai, T. Comparison of the plasma insulin and adiponectin concentrations as metabolic markers in clinically healthy dogs with ageing. J. Anim. Vet. Adv. 11, 971–974 (2012).
-
Schmid, S. M. et al. ; K.E. Creevy. The companion dog as a model for inflammaging: a cross-sectional pilot study. Geroscience 46, 5395–5407 (2024).
-
Lowseth, L. A., Gillett, N. A., Gerlach, R. F. & Muggenburg, B. A. The effects of aging on hematology and serum chemistry values in the beagle dog. Vet. Clin. Pathol. 19, 13–19 (1990).
-
Abinaya, A. & Pasupathi, K. Effect of aging on hematological profile of obese dogs. Int. J. Chem. Stud. 6, 994–996 (2019).
-
Bruno, B. et al. Evaluation of Haemostasis in Dogs affected by Resectable Malignancy. Anim. (Basel) 46(6), (2022).
-
Lee, S. H., Kim, J. W., Lee, B. C. & Oh, H. J. Age-specific variations in hematological and biochemical parameters in middle- and large-sized of dogs. J. Vet. Sci. 21, e7 (2020).
-
Craft, S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheimer Dis. Assoc. Disord. 20, 298–301 (2006).
-
Arcidiacono, B. et al. A. Brunetti. Insulin resistance and cancer risk: an overview of the pathogenetic mechanisms. Exp Diabetes Res. 789174 (2012) (2012).
-
Adin, C. A. & Gilor, C. The Diabetic Dog as a translational model for human islet transplantation. Yale J. Biol. Med. 90, 509–515 (2017).
-
Shaw, J. E., Sicree, R. A. & Zimmet, P. Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 (2010).
-
DiNinni, A. & Hess, R. S. Development of a requirement for exogenous insulin treatment in dogs with hyperglycemia. J. Vet. Intern. Med. 38, 980–986 (2024).
-
McKenzie, B. et al. D. Juarez-Salinas. Feeding dogs a high-fat diet induces metabolic changes similar to natural aging, including dyslipidemia, hyperinsulinemia, and peripheral insulin resistance. Am. J. Vet. Res. 85, (2024).
-
Cardoso, M. J. L. et al. E. Yudi Hashizume. Blood Pressure, Serum Glucose, Cholesterol, and Triglycerides in Dogs with Different Body Scores. Vet Med Int. 8675283 (2016). (2016).
-
Almeida, I., Magalhães, S. & Nunes, A. Lipids: biomarkers of healthy aging. Biogerontology 22, 273–295 (2021).
-
Lien, E. C. et al. MG. Effects of aging on glucose and lipid metabolism in mice. bioRxiv. (2023).
-
Carr, M. C. & Brunzell, J. D. Abdominal obesity and dyslipidemia in the metabolic syndrome: importance of type 2 diabetes and familial combined hyperlipidemia in coronary artery disease risk. J. Clin. Endocrinol. Metab. 89, 2601–2607 (2004).
-
Usui, S., Yasuda, H. & Koketsu, Y. Lipoprotein cholesterol and triglyceride concentrations associated with dog body condition score; effect of recommended fasting duration on sample concentrations in Japanese private clinics. J. Vet. Med. Sci. 77, 1063–1069 (2015).
-
Peloquin, M. et al. Juarez-Salinas. Saturated fatty acid concentrations are predictive of insulin sensitivity and beta cell compensation in dogs. Sci. Rep. 14, 12639 (2024).
-
Rebrin, K., Steil, G. M., Mittelman, S. D. & Bergman, R. N. Causal linkage between insulin suppression of lipolysis and suppression of liver glucose output in dogs. J. Clin. Invest. 98, 741–749 (1996).
-
Boden, G. et al. Ruderman. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappab pathway in rat liver. Diabetes 54, 3458–3465 (2005).
-
Groop, L. C. et al. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Invest. 84, 205–213 (1989).
-
Kolka, C. M. et al. Lipid-induced insulin resistance does not impair insulin access to skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 308, E1001–1009 (2015).
-
Khoramipour, K. et al. Adiponectin: Structure, Physiological Functions, Role in Diseases, and Effects of Nutrition. Nutrients 13, (2021).
-
Bik, W. et al. The relationship between adiponectin levels and metabolic status in centenarian, early elderly, young and obese women. Neuro Endocrinol. Lett. 27, 493–500 (2006).
-
Wang, X. et al. Adiponectin improves NF-κB-mediated inflammation and abates atherosclerosis progression in apolipoprotein E-deficient mice. Lipids Health Dis. 15, 33 (2016).
-
Nishida, M., Funahashi, T. & Shimomura, I. Pathophysiological significance of adiponectin. Med. Mol. Morphol. 40, 55–67 (2007).
-
Xia, J. Y. et al. Acute loss of adipose tissue-derived adiponectin triggers immediate metabolic deterioration in mice. Diabetologia 61, 932–941 (2018).
-
Kim, Y. et al. Park. Adiponectin receptor agonist ameliorates cardiac lipotoxicity via enhancing ceramide metabolism in type 2 diabetic mice. Cell. Death Dis. 13, 282 (2022).
-
Muñoz-Prieto, A., Cerón, J. J., Martínez-Subiela, S., Mrljak, V. & Tvarijonaviciute, A. A systematic review and Meta-analysis of serum adiponectin measurements in the Framework of dog obesity. Anim. (Basel) 10, 1650–1665 (2020).
-
Torrente, C. et al. Tvarijonaviciute. Adiponectin as a sepsis biomarker in dogs: diagnostic and prognostic value. Vet. Clin. Pathol. 49, 333–344 (2020).
-
Kim, H. S., Kang, J. H., Jeung, E. B. & Yang, M. P. Serum concentrations of Leptin and Adiponectin in Dogs with Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 30, 1589–1600 (2016).
-
Center, S. A. Interpretation of liver enzymes. Vet. Clin. North. Am. Small Anim. Pract. 37, 297–333 (2007).
-
Lawrence, Y. A. & Steiner, J. M. Laboratory evaluation of the liver. Vet. Clin. North. Am. Small Anim. Pract. 47, 539–553 (2017).
-
Kwo, P. Y., Cohen, S. M. & Lim, J. K. ACG Clinical Guideline: evaluation of abnormal liver chemistries. Am. J. Gastroenterol. 112, 18–35 (2017).
-
Yan, W., Yan, M., Wang, H. & Xu, Z. Associations of serum alkaline phosphatase level with all-cause and cardiovascular mortality in the general population. Front. Endocrinol. (Lausanne). 14, 1217369 (2023).
-
Li, J. W., Xu, C., Fan, Y., Wang, Y. & Xiao, Y. B. Can serum levels of alkaline phosphatase and phosphate predict cardiovascular diseases and total mortality in individuals with preserved renal function? A systemic review and meta-analysis. PLoS One. 9, e102276 (2014).
-
Fontana, L., Partridge, L. & Longo, V. D. Extending healthy life span–from yeast to humans. Science 328, 321–326 (2010).
-
Caputo, M. et al. F. Prodam. Regulation of GH and GH Signaling by nutrients. Cells 10, 1376–1415 (2021).
-
Vitale, G., Pellegrino, G., Vollery, M. & Hofland, L. J. ROLE of IGF-1 system in the modulation of longevity: controversies and New insights from a centenarians’ perspective. Front. Endocrinol. (Lausanne). 10, 27 (2019).
-
Lemaréchal, R. et al. Canine model of human frailty: adaptation of a Frailty phenotype in older Dogs. J. Gerontol. Biol. Sci. Med. Sci. 78, 1355–1363 (2023).
-
Eigenmann, J. E., Amador, A. & Patterson, D. F. Insulin-like growth factor I levels in proportionate dogs, chondrodystrophic dogs and in giant dogs. Acta Endocrinol. (Copenh). 118, 105–108 (1988).
-
Berryman, D. E., Christiansen, J. S., Johannsson, G., Thorner, M. O. & Kopchick, J. J. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm. IGF Res. 18, 455–471 (2008).
-
AKC Dog Breeds. American Kennel Club. (2022). https://www.akc.org/dog-breeds/. Accessed April 18.
Author information
Authors and Affiliations
Contributions
ERR and FC designed the study. ERR, FC, TAC, KMS, JA and SYW managed and conducted the study. MP, JLG, FC, AT, AN, EEM and DJS carried out the analysis of the data. MP, TAC, KMS, JA, SYW, KT, KV, MN, EEM and DJS were involved in study operations and data acquisition. BM, MP, KG, DJS, CLHH and ERR provided intellectual support. BM, MP, JLG, and ERR were involved in writing the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
All author are current or previous employees of Cellular Longevity Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McKenzie, B., Peloquin, M., Graves, J.L. et al. Changes in insulin, adiponectin and lipid concentrations with age are associated with frailty and reduced quality of life in dogs.
Sci Rep 15, 5380 (2025). https://doi.org/10.1038/s41598-025-89923-z
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-89923-z
This post was originally published on this site be sure to check out more of their content.