Abstract
Duchenne muscular dystrophy (DMD) is a severe neuromuscular disease due to loss of dystrophin, leading to progressive muscle wasting and physical inactivity. In this pilot study, we studied the effect of daily supplementation of the anabolic substrate beta-hydroxy-beta-methylbutyrate (HMB) on whole body protein and amino acid kinetics using novel isotope methods and daily activity in a canine model of DMD. Six DMD dogs were administered 3 g daily of HMB or placebo for 28 days according to a randomized, placebo-controlled, double-blinded crossover design. We measured pre- and post-intervention daily activity, biochemistry markers, and whole-body amino acid kinetics. We tracked daily activity with an activity monitoring device and measured plasma creatine kinase and standard clinical biochemistry panels to monitor muscle and organ function. To calculate whole body and intracellular amino acid production, we administered in the postabsorptive state an IV stable isotope solution containing 20 amino acid tracers. We collected blood before and six times after until two hours post tracer pulse administration and measured amino acid enrichments and concentrations by LC–MS/MS, subsequently followed by (non) compartmental modeling of the decay enrichment curves. Results were expressed as mean with 95% CI. Whole body production, plasma concentrations, and intra-/extracellular compartmental analyses of various amino acids were attenuated in HMB-dosed DMD dogs. Specifically, the plasma concentration of hydroxyproline (marker of collagen breakdown) was significantly higher in the placebo group compared to the HMB group. The intra- and extracellular pool sizes and flux between the two compartments of hydroxyproline was reduced in HMB treated dogs. DMD dogs treated with HMB as compared to placebo had a respective 40% increase in exertional (play) (951 [827, 1075] versus 569 [491, 647]; p < 0.0001) and 10.5% increase in non-exertional (active) activity (15,366 [14742, 15990] versus 13,806 [13148,14466]; p = 0.0016). In addition, a 6% reduction was found in rest time after HMB supplementation compared to placebo (23,857 [23,130, 24,584], versus 25,363 [24500, 26225]; p = 0.0122). Creatine kinase was not statistically different between groups. We did not observe any adverse clinical or biochemical-related effects of HMB dosing. Daily HMB supplementation in DMD dogs can safely and positively influence protein and amino acid metabolism and improve overall daily activity.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked inherited disorder due to out-of-frame mutations in the DMD gene, leading to dystrophin deficiency and a progressive degeneration of skeletal and cardiac muscle1. In humans, it leads to muscle wasting, loss of ambulation and other important motor functions, with death typically occurring by 30–40 years of age.
Development of treatments for DMD first requires assessment in relevant animal models such as the mdx mouse and/or a more clinically relevant large animal model such as the golden retriever muscular dystrophy (GRMD) dog2. In dystrophic mice, dogs, and humans, reduced muscle metabolic enzyme expression and function, abnormal and reduced mitochondria3,4 and abnormal glucose metabolism5,6 were observed because of dystrophin deficiency. Recently, muscle mass and the rates of contractile protein synthesis assessed in urine were lower in boys with DMD, using D3-creatine dilution and oral 2H2O7. Also, plasma amino acid (AA) differed in DMD patients (glutamate and glutamine decreased and the most perturbed AA pathways involved glutamine, alanine, aspartate, and glutamine metabolism) compared to healthy age and BMI matched controls8. However, it was unclear if this was due to disturbed protein/AA metabolism or catabolism and/or due to diet differences8. It should be noted that 22/40 of the DMD patients in this study received corticosteroid treatment, which may have indirectly influenced AA metabolism8.
Beta-hydroxy-beta-methylbutyrate (HMB) is a metabolite of leucine, which can stimulate protein synthesis and reduce protein breakdown in humans9. For instance, we showed that HMB was able to reduce muscle loss and improve function10,11. HMB also mitigated exercise-induced muscle damage and promoted muscle hypertrophy, muscle strength, resistance to fatigue, and muscle regeneration12. Importantly, HMB given up to one of the highest reported doses in the literature, which was 3 g (g) daily (dosages were typically not based on body weight) in humans had minimal to no side effects13,14.
In mdx mice, HMB was added to the daily feed in a 0.5% solution alone or in combination with other supplements, and both showed a reduction in serum creatine kinase (CK), suggesting that HMB had a protective effect on dystrophic muscle15. In DMD boys, an HMB treatment regimen together with other nutraceuticals showed some improvement in timed function measures (6-min walk test and an increase in the daily activity [steps per day]) when given 1.2–1.4 g/day16. However, as HMB was combined with other components, its therapeutic benefit or mechanism of action as a single agent could not be determined.
Therefore, the purpose of this pilot study was to determine if HMB supplementation could improve protein and amino acid metabolism and overall function in DMD dogs.
Methods
We studied six DMD dogs (14.9–20.5 kg body weight; 1 male and 5 females; age ranges from 2.3–2.5 years old) (Table 1). The male dog was hemizygous (dys -) affected while the 5 females were homozygous (dys -/-) affected dogs. Four of the six dogs were siblings (A, D, E, and F). The carrier dogs from a previous study that we used for comparisons were heterozygous females (dys + /-) with ages ranging from 1.3–1.8 years of age. The carriers were housed identically to the DMD dogs in the same sized kennels in the same facility; each group were given identical times to socialize throughout the week while wearing the activity monitors. The carriers were fed a dry kibble diet (Lab Diet) and affected DMD dogs were fed a gruel-based diet (Lab Diet). We would like to note that we only evaluated the affected DMD dogs for plasma amino acids, not the carrier dogs. Carrier dogs were only used to compare activity levels. All dogs were used and cared for according to principles outlined in the National Research Council’s Guide for the Care and Use of Laboratory Animals and reported in accordance with ARRIVE guidelines. Dogs were part of a canine DMD colony maintained at Texas A&M University and approved for use by the Institute for Animal Care and Use Committee (Animal Use Protocol 2021–0105).
Study Design
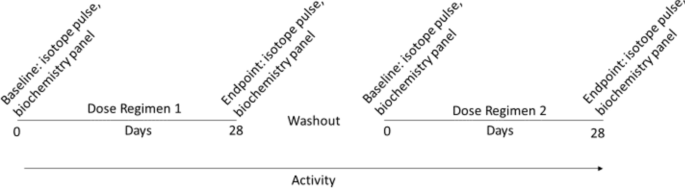
According to a placebo-controlled, double-blinded crossover design (Table 1 and Fig. 1), dogs were randomly assigned to receive daily 3 g of HMB or placebo for 28 days (dosing regimen 1) followed by a wash-out period of 14–21 days. Immediately after the washout period and baseline measurements, dosing regimen 2 started for each dog in which the other nutritional supplement (placebo or HMB) was provided daily for 28 days.
Schematic of the study design. Dogs were dosed in a blinded, placebo-controlled cross-over study for 28 days, washed out for at least 2 weeks, and then crossed over to the opposite dosing regimen (HMB or placebo). Activity was monitored for the entire duration of the cross-over studies. Amino acid isotope pulsing and biochemistry panel analyses were performed at baseline (0d) and endpoint (28d) for each dosing regimen.
Dosing
HMB (Calcium HMB powder; Bulk Supplements)13,14 or placebo was provided in the morning before their meal through the use of gelatin capsules (LFA Capsule Fillers, Fort Worth, Texas), and then Greenies Pill Pockets hickory smoke flavor (Mars Petcare US, Franklin, Tennessee). We initially tried capsules filled with HMB or placebo, but we found that the capsule would get “stuck” in the caudal pharyngeal region due to their dysphagia and the dog would spit it back out (or it would remain in the pharyngeal region). We therefore switched to pill pockets, which the dogs found much more palatable when appropriately mixed with the compound. HMB (3 g/day), which was the largest dose reported in the literature9,12,13,14 and typically not dosed based upon body weight, or placebo (equal amount of beef gelatin) was placed in bottles labeled either “Drug A” or “Drug B” by one lab member (AMR) who held the key for dosing groups. Personnel filling capsules and pillpockets and subsequently dosing were blinded. Dogs were properly identified with microchips, group-housed, and orally dosed in the morning before their meal.
Activity measurements
The dogs were fitted with breakaway cervical collars and equipped with a wearable activity monitoring device (FitBark 2) that was secured to the collar17. In our first publication of activity monitoring with FitBarks17, we detailed the exact type of movements of the carrier and affected DMD dogs that resulted in rest, active, and play. For rest, this included no movement at all or sleep. For active, these movements included lying or sitting and turning the head from side to side. For play, these involved accelerated movements including running, jumping, etc.17. We did not differentiate between different sizes of dogs or differing ages and breed due to our first study limitations17. The device was worn for 24 h per day for the duration of the study and at least five days for familiarization prior to data collection. After familiarization, a pre-dosing, 7-day data collection period was completed to establish a baseline of activity for each animal. Data collection of activity categories were play, active, and rest and reported data were in minutes (min) per day. Play was defined as exertional activity such as jumping, running, and other high-intensity movements. Active was defined as small movements such as standing, walking, and other minor activities. Rest was defined as no movement/sleeping. All data collected from the FitBark 2 monitor was first synced with the FitBark App and then downloaded into an Excel Spreadsheet for analysis.
Clinical biochemistry and whole-body protein and amino acid metabolism
For clinical biochemistry (albumin, albumin:globulin ratio, alkaline phosphatase (ALKP), alanine aminotransferase (ALT), amylase, AST, bilirubin, blood urea nitrogen (BUN), calcium, chloride, cholesterol, creatine kinase, gamma glutamyl-transferase (GGT), glutamate dehydrogenase (GLDH), globulins, glucose, phosphorous, potassium, sodium, and sodium: potassium ratio) and CK assessments, blood draws were performed at baseline for both dosing regimens and after their daily dosing on days 3, 7, 14, 21, and 28. Lithium heparin plasma was used to assess CK levels and biochemistry analytes and sent to the Texas Veterinary Medical Diagnostics Laboratory at Texas A&M University for analysis. Creatine kinase and analytes on the biochemistry panel were determined via colorimetric assays, ion selective electrodes (ISE) (Na, K, and Cl), and calculations (globulins, A:G ratio, Na:K ratio) on a Beckman Coulter DxC 700 AU analyzer through an AAVLD-accredited laboratory18.
To determine whole body production (WBP), plasma concentration, and compartment (intracellular, extracellular, and flux between the two) analyses of a wide variety of AAs, we administered a pulse stable isotope mixture of AAs19,20,21. Isotopes were sourced from Cambridge Isotopes and compounded in the Deutz, ten Have, and Engelen labs19,20,21,22,23,24. After overnight fasting, each dog was weighed. Catheters were placed in both the left and right cephalic veins. An intravenous pulse of a 2.5 ml stable isotope mixture containing 20 different AA isotopes (Table 2) was given in the right cephalic vein. Blood was drawn from the left cephalic vein. Occasionally, a saphenous vein was catheterized when a cephalic catheter was not patent. A baseline blood draw was performed, followed by the stable isotope AA pulse. After the AA pulse was administered, blood was collected at 5, 10, 20, 30, 60, and 115 min. Between blood sampling, catheters were flushed with heparinized saline. Once the last blood draw was performed, the catheters were removed, and the dogs were returned to the home kennel and fed.
Blood processing and amino acid analysis
All blood samples were placed into lithium heparinized or EDTA tubes, immediately put on ice, and then centrifuged (4 °C, 3120 g for 10 min) to obtain plasma. A portion of the plasma was aliquoted with 0.1 vol of 33% (w/w) trichloroacetic acid and stored at -80 °C. Tracer enrichments [tracer: tracee ratio (TTR)] and AA concentrations were analyzed batch-wise by LC–MS/MS via isotope dilution11. Subsequently, WBP and further compartmental analyses of individual AAs were calculated as described20. Detailed methods for AA extraction and analysis by LC–MS/MS can be found in our recent publications22,23.
Biochemical analysis and calculations of metabolic parameters
We natural-log (ln)-transformed the measured TTR decay and then performed ANCOVA linear multi-regression analysis with the covariates groups (body weight [centered], study day, and ln-t [TIME]). The estimated ln (TTR) was converted back by taking its exponential, and then this TTR decay curve was fitted in GraphPad Prism for the two groups (HMB, placebo) with two exponential formulae (TTR = a*EXP(− k1*t) + b*EXP(− k2*t)). The fitted parameters a, b, k1, and k2 were subsequently used to calculate the predicted WBP and the parameters of compartmental analysis with each AA’s 95% confidence interval (CI)23.
The compartmental analysis allowed the calculation of the tracee fluxes between compartments using the information obtained from the tracer pulse. We have identified two compartments that we hypothesized to be the extracellular (Q1) and intracellular (Q2) compartment pools. The amounts of tracee in Q1 and Q2 pools, the flux to/from pool Q2 (F2,1 = F1,2), the flux in/out of pool Q2 (F0,2 = U2), and the irreversible loss were calculated for the dosing groups for every AA. The amount in Q1 relates to differences in the plasma concentration when the Q1 pool volume remains approximately the same. The unit of measure for Q1 and Q2 was μmol and for F0,2, U2, F2,1, F1,2, were μmol/min. Fractional loss = F0,2/(F0,2 + F2,1) and irreversible loss = F0,2* fractional loss. WBP = back to pool 1 = F0,2* (1- fraction loss).
Statistics
Results were expressed as mean [95% CI]. All data were checked for normality and, if necessary, converted logarithmically prior to statistical testing. Statistical analysis of WBP of AAs and the compartmental parameters was performed by ordinary two-way ANOVA on the mean with standard error and n; a Tukey post hoc testing was added to compare differences between the groups. For activity measurements, the area under the curve (AUC) was calculated over each 28-day dosing period for each group (placebo, HMB), and a t-test was used to test for statistical differences between the AUC. Carrier dog 95% CI was calculated via the same parameters from a previous study and used as a reference standard for normal activity17. GraphPad Prism (Version 10) was used for all statistical and data analysis and graph generation. The level of significance was set at 0.05 and was tested two-sided.
Results
Plasma concentrations
Plasma (Table 3) citrulline (CIT; p = 0.016) and proline (PRO; p = 0.028) significantly changed over time but were not different between HMB and control groups. Plasma glycine (p = 0.023) increased in the placebo group regimen by 88%, while there was no change in the HMB-treated group. Hydroxyproline (H-PRO; p = 0.038) likewise increased by 250% in the placebo group but only increased 24% in the HMB-treated group.
Whole body production of AAs
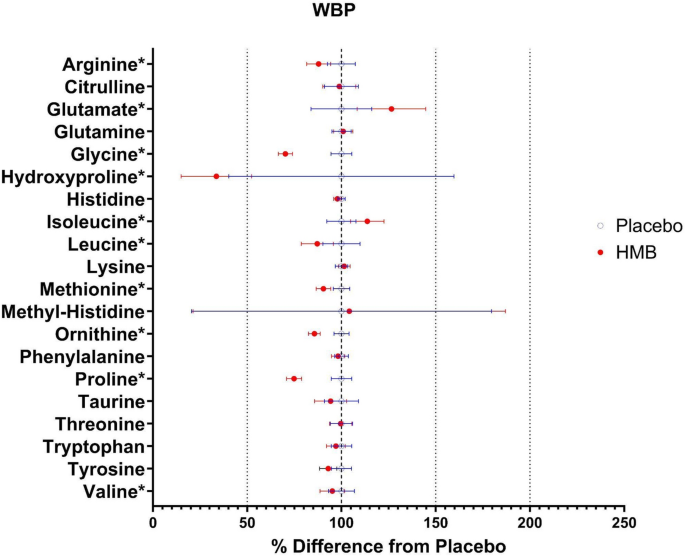
Using our new approach to estimate AA production24, we found the WBP of arginine (ARG; p = 0.0139), glycine (GLY; p < 0.0001), leucine (LEU; p = 0.0477), methionine (MET; p = 0.0014), ornithine (ORN; p = 0.0001), PRO (p < 0.0001), and valine (VAL; p = 0.0352) were lower in the HMB-treated group compared to the placebo group (Fig. 2 and Table 4). In contrast, the WBP of glutamate (GLU; p = 0.0303) and ILE (p = 0.0209) were significantly higher in the HMB group compared to the placebo group.
Forrest plot of whole-body production (WBP) and interconversions of amino acids (AAs). Hydroxyproline, arginine, glycine, leucine, methionine, ornithine, proline, and valine were significantly reduced, while glutamate and isoleucine were increased in the HMB group. Data portrayed as a % difference from the placebo-treated group. Open blue circles were placebo, closed red circles were HMB. Asterisks = statistically different compared to placebo. N = 6 per group.
Intracellular and extracellular compartment pool size of AAs
The extracellular (EC) pool size of glycine (GLY; p = 0.0001), H-PRO (p = 0.0002), and PRO (p = 0.0021) were significantly reduced in the HMB group compared to placebo (Supplemental Table 1). The intracellular (IC) disposal (Supplemental Table 2) of GLY (p = 0.0008) and PRO (p = 0.0151) and intracellular (IC) pool size (Supplemental Table 3) of H-PRO (p = 0.0018) were reduced in the HMB-treated group compared to placebo. Finally, the flux of AAs between the IC and EC compartments were significantly reduced for GLY (p < 0.0001), H-PRO (p < 0.0001), and PRO (p = 0.0002) while GLU (p = 0.0452) was increased in the HMB group compared to placebo (Supplemental Table 4).
Activity
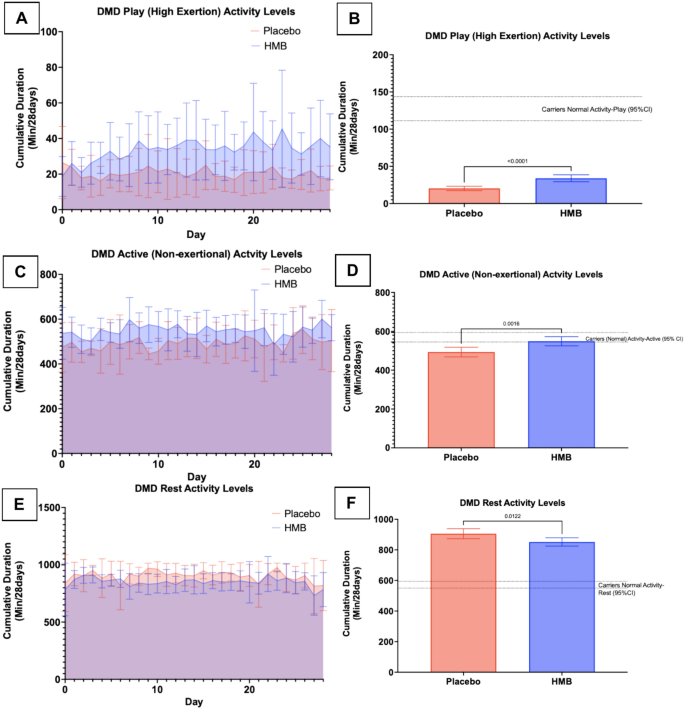
Cumulatively over the 28 day dosing period, the AUC for play activity (total min/28 days), characterized by exertional movements (running, jumping, etc.), increased by 40% when dogs were dosed with HMB vs placebo but remained below exertional play activity for the carrier dogs (Fig. 3A + B; HMB mean = 951; 95% CI [827.3, 1075]; placebo mean = 569; 95% CI [491, 647]; p < 0.0001; N = 6 per group). Dogs’ A, B, D, and F showed the greatest response in exertional play activity when given HMB, while C and E showed minimal to no changes (Supplemental Fig. 1). Active (non-exertional) activity (such as walking and head-turning type movements) AUC saw a 10.5% increase in the HMB group cumulatively in the 28 day dosing period, moving to within the carrier dog active levels (Fig. 3C + D; HMB mean = 15,366 (95% CI [14742, 15990]) placebo mean = 13,806; 95% CI [13148,14466]; p = 0.0016; N = 6 per group). There was a decrease of 6% in the 28-day dosing period AUC for rest activity when dogs were dosed with HMB (Fig. 3E + F; HMB mean = 23,857; 95% CI [23130, 24584]; placebo mean = 25,363; 95% CI [24500, 26225]; p = 0.0122; N = 6 per group).
Activity levels improved for HMB-treated DMD dogs compared to placebo. 3(A) AUC for play (high exertional) activity. 3(B) AUC analysis bar graph for play activity. 3(C) AUC for active (non-exertional) activity. 3(D) AUC analysis bar graph for active activity. 3(E) AUC for rest (not moving/sleeping). 3(F) AUC analysis bar graph for rest. Data were expressed as mean [95% CI] over a 28-day period. N = 6 per group.
Biochemistry panel and physical exam (body weight)
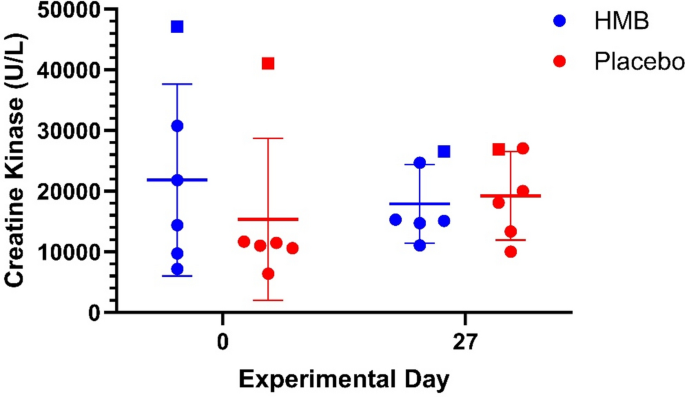
Creatine kinase was followed throughout each dosing regimen and grouped analysis showed a modest 5.6% change in levels after HMB treatment, while CK changed by 30.4% in the placebo regimen, but the results were statistically not significant (Fig. 4).
We also performed biochemistry panels to assess electrolytes, protein levels, and kidney and liver function and did not observe any statistically significant changes with HMB or placebo dosing (Supplemental Table 5). We tracked body weight throughout the dosing regimens and did not detect a noticeable change with either regimen (Supplemental Fig. 2).
Discussion
In the present pilot study, we observed in a placebo-controlled, double blind trial that daily supplementation with 3 g daily of HMB affects protein and AA metabolism and daily activity with a favorable safety profile in a clinically relevant DMD dog model.
In multiple studies in health and disease, including DMD, oral HMB treatment helped to build and/or maintain muscle protein and/or function9,12,14,19. We therefore sought to build upon these studies and give DMD dogs the highest reported dose of HMB (or placebo), 3 g/day, to achieve a possible clinical and metabolic improvement. Our results are in line with what was previously observed and add to these observations that also protein and AA metabolism were changed. However, we acknowledge that further clinical studies are needed to establish long term benefits. We also would like to note that we chose to use the calcium-HMB form as opposed to the free HMB form, as most human studies have used the calcium form9,12,13,14
Amino Acid Kinetics
Stable tracer infusions are used to measure the appearance and disappearance of AAs in plasma, providing valuable insight into their metabolism and also into protein synthesis and breakdown when measured with stable isotopes like phenylalanine and tau-methyhistidine25. Our group has extensively utilized stable tracer infusions both in non-DMD, pre-clinical porcine studies and clinical trials10,11,20,26,27 where we showed protein and other metabolic disturbances associated with critical illness and muscle protein breakdown. In the current study, we analyzed protein breakdown rates in the postabsorptive condition and measured both the whole-body protein breakdown rate with phenylalanine and the muscle protein breakdown rate with tau-methyhistidine. We were not able to observe any change in the protein breakdown rates of these two AAs but observed other significant changes in AAs. As mentioned, our previous studies using phenylalanine and tau-methylhistidine were using non-DMD pre-clinical porcine and human trials and were tailored to these types of studies10,11,20,26,27. Instead, we observed other AA changes such as GLY, GLU, ARG, PRO, H-PRO, LEU, and ILE, which we believe are more in line with changes that occur in dystrophic muscle, as discussed below.
Muscle protein breakdown stimulates an increase in WBP of other non-essential AAs such as GLY and GLU10. In mdx skeletal muscle examined with magnetic resonance spectroscopy, GLY and MET were decreased while GLU, GLN, IlE, and ALA were increased28,29. In two other studies using nuclear magnetic resonance in mdx mouse muscle, DMD myoblasts, and DMD/BMD tissue, researchers showed reduced MET, GLY, GLU, ALA, and taurine30 and increased HIS31. In a recent study, taurine supplementation improved mdx muscle function and reduced inflammation32. On the other hand, GLY remained unaltered in DMD patient urine samples after corticosteroid treatment33. Other studies revealed enhanced therapeutic response when GLY was administered with stem cell, exon skipping, and corticosteroid therapies34,35,36 while L-CIT treatment increased ARG metabolism pathways (as measured by GC–MS) in Becker MD patients37. Other DMD studies administered GLN, amino acid mixtures, or LEU isotopes and revealed reduced WBP of GLU while LEU WBP and oxidation were increased38,39. In our HMB-treated DMD dog group, WBP, EC compartment analysis, IC disposal, and flux between IC and EC compartments of GLY and GLU were also respectively altered compared to placebo. Taken together, there appears to be a species, tissue, time point, and therapeutic response differences in AA levels in dystrophic subjects, most likely reflecting the amount of muscle wasting/protein breakdown and treatment status.
However, when analyzing the production of AAs not directly related to protein breakdown, we found that PRO was altered. In our current study, WBP, IC disposal, EC compartment, and flux between IC and EC compartment pools of PRO were significantly reduced in HMB-treated DMD dogs. Proline is a metabolite of ARG and is hydroxylated to H-PRO post-translationally. The latter is used as a surrogate biomarker for fibrosis in DMD, and several therapeutic intervention studies have documented reduced H-PRO levels in muscle40,41,42. Proline was increased in dystrophic skeletal muscle tissue in a separate study of non-treated GRMD dogs43. We have recently demonstrated that WBP of H-PRO is seen with protein breakdown in non-DMD human populations10. While the WBP of H-PRO was statistically not significant in the HMB treatment group of the current study, plasma concentration, EC compartment, IC compartment, and the flux between IC to EC compartment were significantly reduced. Although promising, further studies are needed to determine if this altered H-PRO metabolism can lead to reduced overall collagen and fibrotic pathway metabolism and a reduction in fibrosis in HMB-treated dystrophic muscle.
The conversion of glutamine (GLN) to CIT to ARG leads to nNOS production, and in DMD, nNOS expression and function are reduced due to dystrophin deficiency44. Dystrophic boys and mice had increased plasma ARG levels, while GRMD dogs had increased ARG metabolites in skeletal muscle43,45. Mdx mice also had reduced plasma GLN and ORN levels45, while in DMD serum, GLN remained unchanged, but muscle GLN was reduced31,46. We also found a reduction in the WBP of ARG with HMB treatment in DMD dogs. However, plasma GLN remained unchanged in all analyses while WBP of LEU and ORN were reduced with HMB treated DMD dogs, in keeping with a reduction in WBP of the ARG metabolism pathway (ORN) and essential AAs (LEU). Leucine was of particular interest since it is known to stimulate protein synthesis in skeletal muscle. As such, it is reasonable to believe that preventing LEU breakdown could enhance protein synthesis in HMB-treated DMD dogs. In addition, in the liver, HMB is produced from KIC, the transamination product of leucine. Therefore, administration of a large concentration of exogenous HMB (3 g/day) may signal negative feedback to reduce endogenous WBP of leucine and possibly valine. Isoleucine is another branched chain AA and is heavily involved with glucose uptake in muscle47. We have shown that the glucose transporter 4 (GLUT-4) is overactive at the dystrophic muscle membrane, thereby leading to increased glucose and glucose analogue uptake, presumably as a compensatory mechanism in dystrophic muscle6. Therefore, the increased isoleucine WBP may be a mechanism why GLUT-4 is overactive at the dystrophic myofiber membrane.
Activity measurements.
We have recently shown that in canine DMD, daily activity levels are highly correlated with isometric muscle strength and eccentric contraction decrement and, therefore, can be used to track response to therapeutic intervention17,48. We further found that daily activity improved in DMD dogs receiving EDG-5506, a fast-twitch myosin modulator48, which is currently being evaluated in clinical trials. Therefore, we believe that tracking daily activity provides a minimally invasive biomarker of muscle strength. Most of the dogs in the present study responded to HMB by increasing their exertional and non-exertional activity and reducing their rest time. It is noteworthy to mention that exertional activity in HMB treated dogs was still markedly below carrier dogs (phenotypically normal), while non-exertional activity reached the level of the carriers. Rest time in HMB treated dogs also did not reach the level of carrier dogs. There was variation in increase of exertional play activity time between HMB-treated dogs, which likely can be attributed to individual phenotype, behavior, and overall response to therapy. For example, Dog C began the study with the highest overall activity levels, especially in exertional play, which likely alludes to why the HMB dosing had a lesser effect on its daily activity measurements. Dog C was also the largest of the DMD dogs and therefore, its mg/kg body weight dosage of HMB was less (146 mg/kg) compared to others. Dog E began the study with the lowest play activity levels overall. This, combined with its less playful personality and also being an overall non-responder to HMB therapy could be reasons for the minimal increase in play activity minutes per day. On the other hand, there is evidence that HMB improves mitochondrial function in myocytes and increases fat oxidation49,50. which might positively influence physical activity but more research is needed to confirm this in our dogs. The outcome of a significant increase in high-level activity in dogs’ A, B, D, and F and a reduction in their rest time are thus reasonably confirmed as a result of a reduction in WBP and other important compartment changes in AAs due to HMB treatment. It should be noted that the carrier dogs were 1.3–1.8 years of age, while the DMD dogs were 2.3–2.5 years of age. In addition to the genotype and phenotype differences due to the disease, the younger age range of the carriers may have led to higher levels of overall activity. Another factor to consider would be the sampling frequency of the FitBark devices and whether they adequately detected movements in a certain time frame. Finally, it should be noted that increases in activity might not correlate with functional improvements in DMD with HMB treatment, as prolonged use could potentially exacerbate the phenotype as the muscles are being used more.
Creatine kinase analysis and safety.
Creatine kinase is used as a serological biomarker of muscle damage/leakage in DMD, and we have used the enzyme levels to longitudinally follow micro-dystrophin gene therapy51 and small molecule treatment (EDG-5506) in affected dogs48. When mdx mice were dosed with HMB or HMB + other supplements, they showed reduced CK levels15. We did not observe statistically significant changes in CK, most likely due to the short nature of the treatment regimen and minimal time for HMB to exert its beneficial effects on protein metabolism. In addition, the increased activity observed in the HMB group could have led to subsequently higher CK levels, thereby leading to the statistically insignificant results when compared to placebo. There were no adverse events associated with HMB (or placebo) dosing nor were there any negative biochemical effects identified in the biochemistry panels. There was no body weight loss or other clinical abnormalities, further confirming HMB is safe at the given dosages.
In our study, we observed with 3 g daily of HMB treatment improved daily activity and for the first time, protein/AA metabolism improvements and a favorable safety profile in a clinically relevant DMD dog model. Pharmacological therapies with corticosteroids are still considered the gold standard of care as they temporarily improve muscle function and delay the loss of ambulation in DMD boys52. The dissociative steroid vamorolone (recently approved by the Food and Drug Administration) also improves muscle function while minimizing some of the negative side effects associated with traditional corticosteroids53. Despite temporarily improving muscle function, other therapies are needed to maintain and build muscle mass. Further studies are needed to determine if HMB can help fill this void when given in conjunction with other standard-of-care therapies.
Our study had some limitations. As we only were able to study six dogs with DMD, we chose to use the cross-over design. Although the power of such a study size is lower, we still were able to observe some statistical differences in the HMB treated group. We also are not sure what the effect is of studying a cohort of mainly sibling DMD dogs. We also provided the HMB dosage once daily. The pharmacokinetic profile of HMB requires that it is provided in two dosages/day. As we were able to only measure muscle function by activity measurements and did not take muscle biopsies to detect histopathological changes/protein synthesis changes, we could not detect true functional increases in muscle strength or improvements in histology. Future studies should assess in vivo isometric/eccentric muscle testing and muscle biopsies to study more in-depth outcome measures in a longer 16-week, placebo-controlled trial.
In conclusion, HMB can improve daily activity and WBP, plasma concentrations, and compartmental metabolism of some AAs, while exhibiting a safe dosing profile at the highest reported dose in the literature.
Data availability
All data will be provided upon reasonable request. To make a data request, please contact Dr. Peter Nghiem at pnghiem@tamu.edu.
References
-
Hoffman, E. P., Brown, R. H. & Kunkel, L. M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 219(2), 402–414 (1987).
-
Kornegay, J. N. et al. Pharmacologic management of Duchenne muscular dystrophy: target identification and preclinical trials. ILAR J. 55(1), 119–149 (2014).
-
Chen, Y. W., Zhao, P., Borup, R. & Hoffman, E. P. Expression profiling in the muscular dystrophies: identification of novel aspects of molecular pathophysiology. J. Cell. Biol. 151(6), 1321–1336 (2000).
-
Rayavarapu, S. et al. Identification of disease specific pathways using in vivo SILAC proteomics in dystrophin deficient mdx mouse. Mol. Cell. Proteomics. 12(5), 1061–1073 (2013).
-
Nghiem, P. P. et al. Changes in Muscle Metabolism are Associated with Phenotypic Variability in Golden Retriever Muscular Dystrophy. Yale J. Biol. Med. 90(3), 351–360 (2017).
-
Schneider, S. M. et al. Glucose Metabolism as a Pre-clinical Biomarker for the Golden Retriever Model of Duchenne Muscular Dystrophy. Mol. Imaging Biol. 20(5), 780–788 (2018).
-
Evans, W. J. et al. Profoundly lower muscle mass and rate of contractile protein synthesis in boys with Duchenne muscular dystrophy. J. Physiol. 599(23), 5215–5227 (2021).
-
Xu, H., Cai, X., Xu, K., Wu, Q. & Xu, B. The metabolomic plasma profile of patients with Duchenne muscular dystrophy: providing new evidence for its pathogenesis. Orphanet. J. Rare Dis. 18(1), 273 (2023).
-
Holecek, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle. 8(4), 529–541 (2017).
-
Deutz, N. E. P. et al. Females have a different metabolic response to critical illness, measured by comprehensive amino acid flux analysis. Metabolism 142, 155400 (2023).
-
Deutz, N. E. P. et al. Comprehensive metabolic amino acid flux analysis in critically ill patients. Clin. Nutr. 40(5), 2876–2897 (2021).
-
Din, U. S. U. et al. A double-blind placebo-controlled trial into the impacts of HMB supplementation and exercise on free-living muscle protein synthesis, muscle mass and function, in older adults. Clin. Nutr. 38(5), 2071–2078 (2019).
-
Wilkinson, D. J. et al. Impact of the calcium form of β-hydroxy-β-methylbutyrate upon human skeletal muscle protein metabolism. Clin. Nutr. https://doi.org/10.1016/j.clnu.2017.09.024 (2018).
-
Kaczka, P. et al. Mechanism of Action and the Effect of Beta-Hydroxy-Beta-Methylbutyrate (HMB) Supplementation on Different Types of Physical Performance – A Systematic Review. J. Hum. Kinet. 68, 211–222 (2019).
-
Payne, E. T. et al. Nutritional therapy improves function and complements corticosteroid intervention in mdx mice. Muscle Nerve. 33(1), 66–77 (2006).
-
Davidson, Z. E. et al. Effect of a multicomponent nutritional supplement on functional outcomes for Duchenne muscular dystrophy: A randomized controlled trial. Clin. Nutr. 40(7), 4702–4711 (2021).
-
Rutledge, A. M. et al. Comprehensive assessment of physical activity correlated with muscle function in canine Duchenne muscular dystrophy. Ann. Phys. Rehabil. Med. 65(5), 101611 (2022).
-
Pincus M.R., McPherson R.A., Bock J. Chemical Basis for Analyte Assays and Common Interferences. Henry’s Clinical Diagnosis and Management by Laboratory Methods. Elsevier. Ch 28. 453–466.e1.
-
Engelen, M. P. K. J. & Deutz, N. Is β-hydroxy β-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations?. Curr. Opin. Clin. Nutr. Metab. Care. 21(3), 207–213 (2018).
-
Deutz, N. E. et al. Reduced mortality risk in malnourished hospitalized older adult patients with COPD treated with a specialized oral nutritional supplement: Sub-group analysis of the NOURISH study. Clin. Nutr. 40(3), 1388–1395 (2021).
-
Kirschner, S. K. et al. Short-chain fatty acid production in accessible and inaccessible body pools as assessed by novel stable tracer pulse approach is reduced by aging independent of presence of COPD. Metabolism https://doi.org/10.1016/j.metabol.2023.155399 (2023).
-
Deutz, N. E. P. et al. Metabolic phenotyping using kinetic measurements in young and older healthy adults. Metabolism 78, 167–178 (2018).
-
Kang M., et al., Aging induced changes in arginine metabolism is dependent on sex, Clinical Nutrition ESPEN. Volume 58, Pages 487–488, ISSN 2405–4577. (2023).
-
Deutz, N. E. P. & Engelen, M. P. K. J. Compartmental analysis: a new approach to estimate protein breakdown and meal response in health and critical illness. Front. Nutr. 11, 1388969 (2024).
-
Wolfe R.R. and Chinkes, D.L. Isotope tracers in metabolic research: principles and practice of kinetic analysis. New York, Wiley: 1–274. (2005).
-
Ten Have, G. A. M., Deutz, R. C., Engelen, M. P. K. J., Wolfe, R. R. & Deutz, N. E. P. Characteristics of a Pseudomonas aeruginosa induced porcine sepsis model for multi-organ metabolic flux measurements. Lab Anim. 52(2), 163–175 (2018).
-
Kang, M. C., Deutz, N. E. P., Kirschner, S. K. & Engelen, M. P. K. J. Metabolic kinetics and muscle and brain health markers in older adults, and the role of age and presence of chronic morbidities: A large cross-sectional cohort study. Clin. Nutr. 43(12), 36–47 (2024).
-
Martins-Bach, A. B., Bloise, A., Vainzof, M. & Rahnamaye Rabbani, S. Metabolic profile of dystrophic mdx mouse muscles analyzed with in vitro magnetic resonance spectroscopy (MRS). Magn. Reson. Imaging. 30(8), 1167–1176 (2012).
-
Lorena, M. D. S. V. et al. Biomarkers for Duchenne muscular dystrophy progression: impact of age in the mdx tongue spared muscle. Skelet. Muscle. https://doi.org/10.1186/s13395-023-00325-z (2023).
-
Panza, E. et al. Duchenne’s muscular dystrophy involves a defective transsulfuration pathway activity. Redox Biol. 45, 102040 (2021).
-
Srivastava, N. K., Yadav, R., Mukherjee, S. & Sinha, N. Perturbation of muscle metabolism in patients with muscular dystrophy in early or acute phase of disease: In vitro, high resolution NMR spectroscopy-based analysis. Clin. Chim. Acta. 478, 171–181 (2018).
-
Terrill, J. R., Pinniger, G., Graves, J. A., Grounds, M. D. & Arthur, P. G. Increasing taurine intake and taurine synthesis improves skeletal muscle function in the mdx mouse model for Duchenne muscular dystrophy. J Physiol. 594(11), 3095–3110 (2016).
-
Kawai, H. et al. Decrease in urinary excretion of 3-methylhistidine by patients with Duchenne muscular dystrophy during glucocorticoid treatment. J. Neurol. 240(3), 181–186 (1993).
-
Lin, C. et al. Glycine Enhances Satellite Cell Proliferation, Cell Transplantation, and Oligonucleotide Efficacy in Dystrophic Muscle. Mol. Ther. 28(5), 1339–1358 (2020).
-
Lin, C. et al. Cardio-respiratory and phenotypic rescue of dystrophin/utrophin-deficient mice by combination therapy. EMBO Rep. 23(6), e53955 (2022).
-
Ham, D. J. et al. Glycine administration attenuates progression of dystrophic pathology in prednisolone-treated dystrophin/utrophin null mice. Sci. Rep. 9(1), 12982 (2019).
-
Hanff, E. et al. Effects of single and combined metformin and L-citrulline supplementation on L-arginine-related pathways in Becker muscular dystrophy patients: possible biochemical and clinical implications. Amino Acids 50(10), 1391–1406 (2018).
-
Hankard, R., Mauras, N., Hammond, D., Haymond, M. & Darmaun, D. Is glutamine a “conditionally essential” amino acid in Duchenne muscular dystrophy?. Clin. Nutr. 18(6), 365–369 (1999).
-
Mok, E. et al. Oral glutamine and amino acid supplementation inhibit whole-body protein degradation in children with Duchenne muscular dystrophy. Am. J. Clin. Nutr. 83(4), 823–828 (2006).
-
Bish, L. T. et al. Chronic losartan administration reduces mortality and preserves cardiac but not skeletal muscle function in dystrophic mice. PLoS One. https://doi.org/10.1371/journal.pone.0020856 (2011).
-
Gosselin, L. E., Williams, J. E., Personius, K. & Farkas, G. A. A comparison of factors associated with collagen metabolism in different skeletal muscles from dystrophic (mdx) mice: impact of pirfenidone. Muscle Nerve. 35(2), 208–216 (2007).
-
Smith, L. R. et al. Increased collagen cross-linking is a signature of dystrophin-deficient muscle. Muscle Nerve. 54(1), 71–78 (2016).
-
Abdullah, M. et al. Non-Targeted Metabolomics Analysis of Golden Retriever Muscular Dystrophy-Affected Muscles Reveals Alterations in Arginine and Proline Metabolism, and Elevations in Glutamic and Oleic Acid In Vivo. Metabolites. 7(3). (2017).
-
Boehler, J. F. et al. Membrane recruitment of nNOSµ in microdystrophin gene transfer to enhance durability. Neuromuscul. Disord. 29(10), 735–741 (2019).
-
Tsonaka, R. et al. Longitudinal metabolomic analysis of plasma enables modeling disease progression in Duchenne muscular dystrophy mouse models. Hum. Mol. Genet. 29(5), 745–755 (2020).
-
Spitali, P. et al. Cross-sectional serum metabolomic study of multiple forms of muscular dystrophy. J. Cell Mol. Med. 22(4), 2442–2448 (2018).
-
Doi M., et al. Isoleucine, a blood glucose-lowering amino acid, increases glucose uptake in rat skeletal muscle in the absence of increases in AMP-activated protein kinase activity. J. Nutr. 135(9):2103–8. (2005).
-
Russell, A. J. et al. Modulating fast skeletal muscle contraction protects skeletal muscle in animal models of Duchenne muscular dystrophy. J. Clin. Invest. 133(10), e153837 (2023).
-
Zhong, Y. et al. β-hydroxy-β-methylbutyrate (HMB) improves mitochondrial function in myocytes through pathways involving PPARβ/δ and CDK4. Nutrition 60, 217–226 (2019).
-
Sadeghi B., et al. Effects of β-hydroxy-β-methylbutyrate (HMB) supplementation on lipid profile in adults: a GRADE-assessed systematic review and meta-analysis of randomized controlled trials. Front. Nutr. (2024).
-
Birch, S. M. et al. Assessment of systemic AAV-microdystrophin gene therapy in the GRMD model of Duchenne muscular dystrophy. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.abo1815 (2023).
-
Biggar, W. D., Skalsky, A. & McDonald, C. M. Comparing Deflazacort and Prednisone in Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 9(4), 463–476 (2022).
-
Guglieri, M. et al. Efficacy and Safety of Vamorolone vs Placebo and Prednisone Among Boys With Duchenne Muscular Dystrophy: A Randomized Clinical Trial. JAMA Neurol. 79(10), 1005–1014 (2022).
Acknowledgements
We acknowledge the caretakers and veterinary staff of the canine colony.
Author information
Authors and Affiliations
Contributions
PPN, AR, KT, CK, MP, SA, VD, GAMTH, MPKJE, and NEPD were involved with experimental design, experimental deployment, recording and analysis of data, and manuscript writing. CVS and MLM were involved with recording and analysis of data and manuscript writing.
Corresponding author
Ethics declarations
Competing interests
PPN is a paid consultant of Agada Biosciences, a scientific advisory board member of Bioramics, LLC, and has an equity stake in SCRM-Bio. The other authors do not report any competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nghiem, P.P., Rutledge, A.M., Tehas, K. et al. Beta-hydroxy-beta-methylbutyrate (HMB) improves daily activity and whole-body protein metabolism in Duchenne muscular dystrophy dogs: a pilot study.
Sci Rep 15, 4026 (2025). https://doi.org/10.1038/s41598-025-88651-8
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-88651-8
Keywords
This post was originally published on this site be sure to check out more of their content.