Abstract
Insulinoma is the most common pancreatic tumor diagnosed in dogs. This study aimed to report incidence risk, breed predispositions and other demographic risk factors for insulinoma diagnosed in dogs under primary veterinary care in the UK. The VetCompass Program supports research on anonymized electronic health records (EHRs) from dogs under UK veterinary care. This study included all VetCompass EHRs from dogs under primary veterinary care during 2019. Multivariable logistic regression analysis was used to evaluate demographic risk factors for insulinoma diagnosis. Of 2,250,741 study dogs, 278 were confirmed as insulinoma cases at any date. The estimated 2019 incidence risk was 0.003% (95% CI 0.002–0.004%). Compared to crossbreeds, predisposed breeds included Dogue de Bordeaux, German Pointer, Flat Coated Retriever, Boxer and West Highland White Terrier. The Labrador Retriever showed decreased odds for insulinoma diagnosis. Additionally, being a terrier breed and being a breed predisposed to other endocrine cancers were associated with increased odds for insulinoma diagnosis. Other risk factors associated with increased odds for insulinoma diagnosis included being female neutered, being 9 – <15 years of age>
Introduction
Insulinoma is the most common neuroendocrine tumor of the pancreas in dogs and humans1,2. In both species, neoplastic insulinoma β-cells secrete insulin in an uncontrolled fashion, leading to hyperinsulinemia-induced hypoglycemia. Human malignant insulinoma and spontaneous canine insulinoma share similar histological features, molecular characteristics and responses to multimodal treatment protocols3,4,5,6,7. Hence, canine insulinoma has been validated as a research model for human malignant insulinoma7. The advantages of using a canine insulinoma model over a rodent model include the fact that dogs more closely represent the genetics of humans than rodents and develop insulinomas spontaneously in contrast to genetically induced insulinomas in rodents. Additionally, dogs share their living environment with humans and are exposed to similar environmental factors. Moreover, spontaneous insulinomas in dogs provide a model with intact host immunity, as well as natural tumor heterogeneity and microenvironment. However, further research into the genetic background of these tumors is needed to determine whether they share the same drivers as those found in human patients8.
In humans, the reported incidence of insulinoma ranges from 1 to 4 cases per million population per year9. The incidence risk for canine insulinoma has not been formally reported but has been suggested to be higher than for human insulinoma6. Despite this, canine insulinoma is considered an under-recognized disease in primary care veterinary practice because clinical hypoglycemia can be intermittent and non-specific, and can mimic clinical signs of other endocrine, neurological and/or cardiovascular diseases10,11.
Although most insulinoma in humans occur sporadically, 5–10% of human cases are linked with Multiple Endocrine Neoplasia type 1 (MEN1), a genetic syndrome characterized by simultaneous occurrence of neoplasms in multiple endocrine organs. MEN1 is characterized by the simultaneous occurrence of at least two endocrine tumors arising from either the pancreas, parathyroid, and/or pituitary glands12. In veterinary medicine, MEN is preferably called Concurrent Endocrine Neoplasia (CEN), although reported instances of this syndrome in dogs are rare13.
In humans up to 30% of pancreatic neuroendocrine neoplasms (panNENs) are functional and the clinical syndrome is related to the specific hormone overproduction, with the remainder of panNENs being non-functional14. Several risk factors are reported as associated with the occurrence of human sporadic panNENs. Personal history of diabetes mellitus and a family history of cancer, and heavy smoking and heavy alcohol consumption have been linked with increased risk of pancreatic neuroendocrine tumors in various single institutional case-control studies15,16,17,18. In a multinational European case-control study, only non-recent onset diabetes mellitus was associated with increased occurrence of pancreatic neuroendocrine tumors19. However, a major limitation of these studies is that they focused on risk factors for pancreatic neuroendocrine tumors in general, with only small subgroups of insulinoma patients included within those overall study populations, precluding conclusions regarding specific risk factors for insulinoma. Only one study performed in a Chinese population looked specifically at insulinoma, and reported a family history of pancreatic endocrine tumors or other cancers and living in a rural area as risk factors for insulinoma20.
The literature on insulinoma in dogs is dominated by case reports and case series that focused on clinical findings and outcomes, with very little published work that included large numbers of dogs to explore breed predisposition and other risk factors. Most cases of insulinoma in these case series were diagnosed in medium and large breed dogs, although some cases were also described in small breed dogs. Apparently overrepresented breeds in these studies included Boxer, terrier and retriever breeds, although a clear breed predisposition for insulinoma has not been established10,21,22,23,24,25. Similarly, no sex predisposition has been reported and no data are available on other risk factors for the diagnosis of insulinoma in dogs22. The reported median age at insulinoma diagnosis ranged from nine to eleven years10,11,21,22,23,24,25.
‘Big Data’ resources and data-sharing initiatives have proven hugely valuable in advancing the understanding of epidemiology of uncommon and rare diseases26,27. The VetCompass Program, a UK nationwide veterinary surveillance system, shares anonymized veterinary electronic health records (EHRs) from over 14 million dogs and has been validated as a valuable research resource by over 140 peer reviewed publications to date28. Using VetCompass EHRs, the current study aimed to report the annual (2019) prevalence and incidence risk as well as demographic risk factors for insulinoma diagnosis in dogs under primary veterinary care in the UK. The study placed particular focus on associations with breed. These results could assist veterinary practitioners by providing a stronger evidence base to improve diagnosis and management of insulinoma in dogs.
Results
Demography
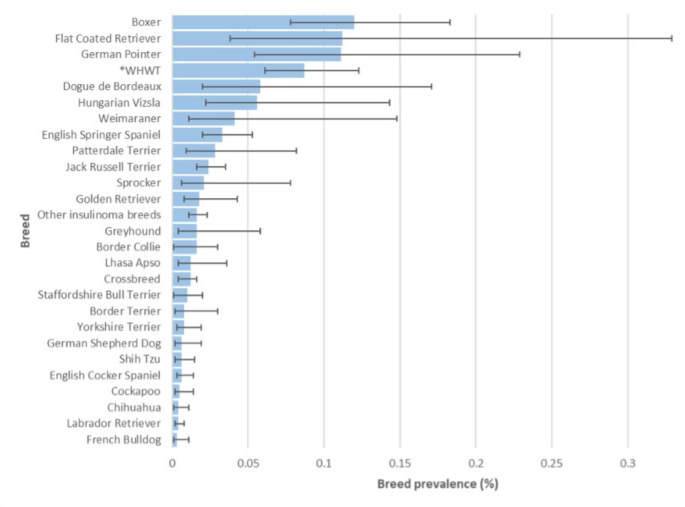
From the study population of 2,250,741 dogs under primary veterinary care in 2019, 1,881 (0.08%) candidate insulinoma cases were identified. Manual review of all candidate cases confirmed 278 (14.8%) as insulinoma cases that met the inclusion criteria at any date in the available EHRs up to August 31, 2023, resulting in an available-lifetime prevalence for insulinoma diagnosis of 0.012% (95% CI 0.011–0.014). The initial insulinoma diagnoses of the 278 cases were made between 2015 and 2023 and within the 278 cases, 110 cases were diagnosed at any date up to 31 December 2019, resulting in an annual (2019) prevalence of 0.004% (95% CI 0.003–0.005). Sixty-three cases were first diagnosed in 2019, giving an annual (2019) incidence risk for insulinoma diagnosis of 0.003% (95% CI 0.002–0.004). Of breeds with ≥ 2 insulinoma cases, the breeds with the highest available-lifetime prevalence were Boxer (n = 21, 0.12%, 95% CI 0.08–0.18%), Flat Coated Retriever (n = 3, 0.11%, 95% CI 0.04–0.33%), German Pointer (n = 7, 0.11%, 95% CI 0.05–0.23%) and West Highland White Terrier (n = 31, 0.09%, 95% CI 0.06–0.12%) (Fig. 1).
Of the 278 insulinoma cases diagnosed up until August 31, 2023, 123 (44.24%) were male (with 52.03% neutered), 153 (55.04%) were female (with 73.20% neutered), and the sex was not recorded in 2 cases (0.72%). Age at the time of diagnosis was available for 268/278 (96.40%) dogs. The median age at first diagnosis was 10.4 years (interquartile range [IQR] 8.7–12.2, range 3.2–16.2). Median adult bodyweight was available for 252/278 (90.65%) dogs. The median of the median adult bodyweight was 18.9 kg (IQR 9.3–28.4, range 3.4–68.3). The most commonly diagnosed breeds with insulinoma were crossbreed, West Highland White Terrier, and Jack Russell Terrier (Table 1).
Of the random sample of 750,000 non-insulinoma cases, 388,113 (51.75%) were male (with 42.80% neutered), 355,532 (47.40%) were female (with 44.80% neutered) and the sex was not recorded in 6,355 cases (0.85%). The median age at 31 December 2019 was available for 744,152/750,000 (99.22%). The median age at 31 December 2019 was 5.2 years (IQR 2.3-9.0, range 0.0-24.8). The median adult bodyweight was available for 513,735/750,000 (68.50%) dogs. The median of the median adult bodyweight was 13.7 kg (IQR 8.4–24.4, range 0.4–95.0). The most common breeds among non-insulinoma cases were crossbreed, Labrador Retriever, and Jack Russell Terrier (Table 1).
Risk factor analysis
Univariable binary logistic regression modelling identified 14 variables liberally associated with insulinoma diagnosis at any date in the available records up to August 31, 2023 (P < 0.20): sex/neuter, age, median adult bodyweight, purebred status, breed, median adult bodyweight in relation to the median for the sex/breed, terrier breed, breed predisposed for mammary gland cancer, breed predisposed for parathyroid cancer, breed predisposed for thyroid cancer, breed predisposed for parathyroid and/or thyroid cancer, breed predisposed for parathyroid and/or thyroid and/or pituitary/adrenal cancer, breed predisposed for reproductive organ cancer and breed predisposed for testicular and/or ovarian cancer (Tables 1 and 2).
The final breed-focused multivariable logistic regression model retained four variables: breed, sex/neuter, age and median adult bodyweight in relation to the median for the sex/breed (Table 3). The area under the Receiver Operating Characteristic (ROC) curve of the final breed-focused model was 0.868, indicating good discrimination. Seven breeds had increased odds for insulinoma diagnosis compared to crossbreed. The Dogue de Bordeaux, German Pointer, Flat Coated Retriever and Boxer showed the highest predisposition. The Labrador Retriever showed decreased odds for insulinoma diagnosis compared to the crossbreed. Increased odds for insulinoma diagnosis was identified for female neutered dogs compared with female entire dogs, dogs with a median adult bodyweight above the median for the sex/breed compared to dogs with a median adult bodyweight at or below the median for the sex/breed, and dogs aged between 9 – <12 years and 12="12" ->
As described in the methods, breed-derived variables were introduced individually to replace breed in the final breed-focused model. Five other variables were identified as significantly associated with insulinoma diagnosis: median adult bodyweight (20 – <30>4).
Discussion
This is the largest study to date to report the frequency and risk factors for diagnosis with insulinoma in dogs under primary veterinary care. Several breed predispositions and other risk factors were identified that can help improve recognition of insulinoma in primary veterinary care and may contribute to translational insulinoma studies that can inform human medicine.
The current study identified an annual prevalence of 0.004% in 2019 for insulinoma in dogs under primary veterinary care in the UK and a corresponding annual incidence risk of newly diagnosed insulinoma of 0.003%. Additionally, we found an available-lifetime prevalence for the diagnosis insulinoma of 0.012%. No previous studies have reported on data from primary veterinary care practices that could be useful for comparison to the current results. Previously, Capodanno et al., 2018 reported that the only academic veterinary referral hospital in the Netherlands recorded 10 new referral cases of canine insulinoma yearly from an overall two million dogs in the Netherlands, but their study was not designed to calculate an annual incidence risk, so direct comparisons between their finding and the current study results cannot be made6. Human studies have reported incidence rates of insulinoma ranging from 0.0 to four cases per million population per year (0.0000-0.0004%), which is ten times lower than reported in the current study for dogs29,30,31. Diagnosing canine insulinoma can be challenging because the clinical signs are often non-specific and can mimic other neurological, endocrine and metabolic conditions10,11. Furthermore, 68Gallium DOTA-(Tyr3)-octreotate positron emission tomography/computed tomography, a highly sensitive and specific imaging technique frequently used in the diagnostic work-up of insulinomas in humans, is not available in veterinary medicine32. Therefore, some or even many true insulinoma cases may have remained unascertained in the current population studied and consequently the current prevalence figures may be an underestimate. Proportional malignancy of human insulinoma is reportedly much lower than for canine insulinoma, with only 5–16% of human insulinoma considered malignant compared to almost all canine insulinoma considered malignant based on their metastatic behavior5,31. A lack of understanding of human malignant insulinoma tumorigenesis can be partly attributed to the low annual incidence rate of human malignant insulinoma. The up to ten times higher estimated annual incidence rate of insulinoma in dogs compared to humans combined with the substantially higher rates of malignancy of insulinoma in dogs underpins the value of spontaneous canine insulinoma as a translational study model for human malignant insulinoma because canine insulinoma samples are more readily available for molecular studies, unlike human malignant insulinoma samples.
The current study identified seven breeds with predisposition for insulinoma diagnosis: Dogue de Bordeaux, German Pointer, Flat Coated Retriever, Boxer, Hungarian Vizsla, West Highland White Terrier and English Springer Spaniel. Among the seven breeds identified with increased odds of insulinoma diagnosis, six breeds demonstrated ultra-predispositions, defined as having over four-times higher odds compared to crossbreed dogs33. Of these ultra-predisposed breeds, the Dogue the Bordeaux, Flat Coated Retriever and German Pointer have not been previously identified as overrepresented for insulinoma10,21,22,23,24,25. The finding of ultra-predispositions for the Boxer and West Highland White Terrier aligns with previous descriptive studies that generally reported these breeds as overrepresented for insulinoma10,21,22,23,24,25. Previous insulinoma studies, however, were descriptive and did not statistically compare breed proportional risk to a control or denominator population, making direct comparison to the current work difficult. The Boxer and Flat Coated Retriever are frequently reported with increased odds for cancers in general so the findings of the current study may represent an extension of this overall cancer predilection34,35,36,37,38,39.
The current study also identified an association between being a terrier breed in general and insulinoma diagnosis. Terrier breeds have previously been reported to cluster in one of the 23 breed clades that were identified in a genomic analysis including 161 breeds that investigated the influence of geographic origin, migration and hybridization on modern dog breed development40. Knowledge of predisposed breeds may aid in insulinoma recognition and contributes to further understanding of canine insulinoma genetics. Especially for a rarely diagnosed disease like canine insulinoma, future genomic studies into genetic variants that contribute to canine insulinoma development could benefit from comparing between breeds that are predisposed to insulinoma versus breeds that are protected against insulinoma. Although the current study did not aim to identify breeds with protection to insulinoma, the Labrador Retriever was identified with relative protection against insulinoma diagnosis amongst those breeds with 2 or more insulinoma cases diagnosed. This finding would need confirmation in future work using a different data source to avoid Type I error (false positive) but, if confirmed, the Labrador Retriever breed could represent a useful negative control for future genetic studies. There may be other breeds with relative protection, especially breeds with no insulinoma diagnosed cases in this population, but relative protection was not the primary focus of the current study. Future breed-focused research, including even greater numbers of cases, could provide new insights on breeds protected for insulinoma diagnosis.
A strong association was identified in the current study between sex/neuter status and insulinoma diagnosis that has not been reported previously. Altered hormonal patterns could explain insulinoma predisposition in female neutered dogs compared to female entire dogs. Female neutered dogs have reduced serum estrogen levels, whereas cyclical estrogen exposure has been reported to be a protective factor against pancreatic neuroendocrine tumorigenesis, although a definitive role for estrogen exposure cannot be established by our data41. Another potential explanation for predisposition of female neutered dogs for insulinoma is that the median age at insulinoma diagnosis was 10 years and the proportion of female dogs that is neutered increases over time. Age was strongly associated with the odds of insulinoma diagnosis. Dogs aged 9 – <15 years had increased odds for insulinoma diagnosis and dogs aged 3="3" ->10,11,21,22,23,24,25,42.
Absolute bodyweight was strongly associated with the odds of insulinoma diagnosis. Dogs with a median adult bodyweight of 20–30 kg had increased odds for insulinoma diagnosis compared with dogs with a median adult bodyweight of < 10 kg. This finding is consistent with previous insulinoma studies that report medium and large breed dogs (> 25 kg) as overrepresented42.
Within breeds, dogs with a median adult bodyweight above the median for the sex/breed had increased odds for insulinoma diagnosis. Previous studies in both humans and dogs have reported associations between higher body condition score and/or obesity and certain types of cancer, including human pancreatic neuroendocrine tumors16,20,43,44. Similarly, the current results could also be interpreted to offer some support for increased risk of insulinoma in overweight dogs. However, caution should be exercised with this interpretation as the current study used the median adult bodyweight compared to the median for the sex/breed and it may be that higher bodyweight reflects larger individual dogs rather than higher body condition score45. Additionally, dogs with insulinoma tend to gain weight after the condition develops due to anabolic effects of insulin on metabolism. Therefore, weight gain leading to a higher median weight compared to the median for the sex/breed could be a consequence rather than a cause of insulinoma42.
Until now, low case numbers in previous works have limited the evidence of CEN in veterinary medicine; only one dog has been reported in the literature to have concurrent insulinoma and pituitary adenoma, resembling human MEN1 syndrome12,13. MEN1 is caused by a germline mutation in the MEN1 gene and is inherited as an autosomal disorder. Although Beatrice et al. (2018) reported only one dog resembling MEN1 syndrome, they did find other combinations of insulinoma with concurrent endocrine tumors in dogs as well, including insulinoma with pheochromocytoma, or adrenocortical hyperplasia13. Additionally, Kiupel et al. (2000) reported an insulinoma, bilateral adrenocortical adenocarcinomas and an aortic paraganglioma in a 12-year-old crossbreed46. In contrast to these sporadic earlier CEN reports based on smaller study populations, the current study benefited from data on a large denominator of dogs, which supported identification of associations between being a breed predisposed for certain types of endocrine cancer and insulinoma diagnosis. The current study demonstrated that breeds predisposed for parathyroid-, thyroid- and parathyroid and/or thyroid cancer had increased odds for insulinoma diagnosis. This association should be interpreted with caution because the current study included insulinoma diagnoses only from dogs in the UK, where genetic bottleneck effects and region-specific breed distributions may influence the pathogenesis of this disease. Furthermore, the lists of endocrine cancer predisposed breeds were not UK specific, but included data from studies that were conducted outside the UK. Additionally, these endocrine cancer predisposed breed lists may have lacked specificity due to reliance on prior studies that used different statistical methods with varying statistical power. Still, the reported association provides a rationale to design future prospective multi-institutional studies investigating the etiopathogenesis of CEN in dogs. These studies should focus on investigating the presence of mutations in the MEN1, NF-1, and TSC1/TSC2 genes, because insulinomas can develop in the context of the genetic tumor syndromes that occur due to mutations in these genes47.
In addition to the limitations discussed above, the current study had some other limitations associated witfh the secondary use of veterinary EHRs as a research resource. These limitations included issues such as missing data and variations in the rigor and quality of the available clinical records. Some of the dogs determined to have insulinomas by a primary care veterinarian may have been misclassified and thus posed a limitation to the study due to possible false positive diagnosis. Additionally, given that only around 30% of UK veterinary practices are collaborating with VetCompass, the current results may not generalize fully to all UK practices, although the UK veterinary practices collaborating with VetCompass include practices of varying sizes, geographical locations and caseloads48. Unmeasured confounding factors such as socio-economic status of owners, regional differences in veterinary care and environmental factors, and differences in physical activity levels of dogs may also have influenced the results of the current study. Finally, this study did not apply corrections for multiple testing in the various analyses carried out, as this was an exploratory study in nature that can support future hypothesis generation but where the current study did not involve testing a predefined hypothesis. It should be noted that failure to correct for multiple testing increases the probability of finding at least one result to be statistically significant just by chance (the problem of multiplicity), which should be considered when interpreting the results49.
In conclusion, this is the first epidemiological study on canine insulinoma in the UK dog population under primary veterinary care and reported an annual incidence risk of 0.003% and an annual prevalence of 0.004%. Nine risk factors were associated with canine insulinoma: breed, sex/neuter, age, median adult bodyweight, median adult bodyweight compared to the median for sex/breed, terrier breed, breed predisposed for parathyroid cancer, breed predisposed for thyroid cancer and breed predisposed for parathyroid and/or thyroid cancer. Dogue de Bordeaux, German Pointer, Flat Coated Retriever, Boxer, Hungarian Vizsla and West Highland White Terrier were identified as breeds with ultra-predisposition for insulinoma diagnosis compared with crossbreeds. These findings of risk factors and breed predispositions can contribute to improved recognition of cases of canine insulinoma and enhance future understanding of insulinoma tumorigenesis.
Methods
The study population included 2,250,741 dogs under primary veterinary care in 2019 at practices across the UK participating within the VetCompass program28. VetCompass collates de-identified EHR data from veterinary practices in the UK for epidemiological research28. Dogs under veterinary care were defined as those with ≥ 1 EHR (free-text clinical note, treatment, or bodyweight) recorded in 2019. Data fields available to VetCompass researchers include a unique animal identifier along with species, breed, date of birth, sex and neuter status and also clinical information from free-form text clinical notes, bodyweight and treatment with relevant dates28. Of the dogs with ≥ 1 EHR recorded in 2019, cohort EHR data recorded up to August 31, 2023 were accessible for the current study.
A retrospective cohort study design was used to estimate the annual (2019) prevalence and annual (2019) incidence risk for diagnosis with insulinoma and to identify associations between demographic risk factors and insulinoma diagnosis at any date in the available clinical records. Based on a preliminary screen suggesting a crude prevalence for insulinoma of 0.01% within VetCompass, power calculation estimated that at least 150,747 dogs were needed to estimate the frequency for a disorder occurring in 0.01% of dogs with 0.005% acceptable margin of error at a 95% confidence level from an estimated national UK population of 8 million dogs50,51. Ethical approval was granted by the RVC Social Science Research Ethical Review Board (SSRERB) (reference number SR2018-1652).
Insulinoma cases were defined as having at least one of the following inclusion criteria:
-
1.
Evidence of a recorded final diagnosis of insulinoma in available EHRs.
-
2.
Histopathological confirmation of insulinoma reported in available EHRs.
-
3.
Concurrent occurrence of blood glucose < 4.2 mmol/L, plasma insulin > 10 µU/mL and matching clinical signs of hypoglycemia in available EHRs.
-
4.
Evidence of concurrent hypoglycemia, a pancreatic mass lesion on diagnostic imaging, matching clinical signs of hypoglycemia and absence of systemic inflammatory response syndrome in available EHRs.
Case-finding involved two phases. Firstly, candidate cases for insulinoma were identified by searching the clinical notes using the search terms: ‘insulinoma*’, ‘diazoxide’, ‘blood insulin’, ‘serum insulin’, ‘octreotide’, ‘streptozocin’ and ‘streptozotocin’ and the treatment fields using the search terms: ‘diazoxide’ and ‘eudemine’. The clinical notes of all candidate cases were manually reviewed to evaluate for case inclusion. Non-cases of insulinoma used in the risk factor analysis included a random sample of 750,000 dogs not identified as candidate cases.
Following data cleaning in Excel (Microsoft Office Excel 2016), statistical analysis used SPSS® Statistics version 29 (IBM®). Breed descriptive information entered by the participating practices was cleaned and mapped to a VetCompass breed list derived and extended from the VeNom Coding breed list that included both recognized purebred breeds and also designer breed terms52. A breed purity (named Purebred) variable categorized all dogs of recognizable breeds as ‘purebred’, dogs with contrived names generated from two or more purebred breed terms as ‘designer’ crossbreds (purposely bred crossbreeds) and dogs recorded as mixes of breeds but without a contrived name as ‘crossbred’53. A Breed variable retained individual pure breeds and designer hybrids represented by ≥ 2 insulinoma cases, along with a grouping of all remaining breeds and also a grouping of general crossbred dogs. This approach was taken to facilitate statistical power for the individual breed analyses54. A terrier variable distinguished terrier breeds assigned to the breed group ‘terrier’ and other breeds as ‘non-terrier’ following a combined classification according to the Kennel Club and VeNom Coding Group, as shown in Supplementary Table 152,53. A list of breeds predisposed for various types of endocrine cancer was created within the current study based on evidence sourced from multiple studies. Studies were selected based on having a reasonable study population size and, preferably, using validated statistical models, implicating sufficient statistical power (Supplementary Table 2). Based on this list, multiple variables were created, including Breed predisposed for parathyroid cancer, Breed predisposed for thyroid cancer, Breed predisposed for parathyroid- and/or thyroid cancer, Breed predisposed for pituitary/adrenal gland cancer, Breed predisposed for parathyroid- and/or thyroid- and/or pituitary/adrenal gland cancer, Breed predisposed for mammary gland cancer, Breed predisposed for testicular/ovarian cancer, Breed predisposed for reproductive endocrine cancer and Breed predisposed for one or multiple types of endocrine cancer.
Sex and neuter status were defined by the final available EHR value and were combined into a single variable for the risk factor analysis. Median adult bodyweight was defined as the median of all bodyweights (kg) values recorded for each dog after reaching 18 months of age and was categorized as: <10>
Lifetime prevalence for insulinoma diagnosis was calculated as the total number of cases at any date in all available EHRs up to August 31, 2023 divided by the total number of dogs in the study. The one-year (2019) period prevalence was estimated by dividing all dogs that met the case definition at any date up to December 31, 2019 by the denominator population described above. The annual incidence risk was calculated as the number of cases newly diagnosed in 2019 divided by the total number of dogs. Breed lifetime prevalence was estimated similarly, by dividing the number of cases of a breed within the lifetime insulinoma group by the number of dogs of that breed within the denominator population. The confidence intervals (CI) for the prevalence and incidence values were derived from standard errors, based on approximation to the binomial distribution. Risk factor analysis included all cases diagnosed at any date to August 31, 2023 (i.e. available lifetime risk) to maximize statistical power. Binary logistic regression modelling was used to evaluate univariable associations between risk factors for diagnosis (sex/neuter, breed, purebred status, median adult bodyweight, age, median adult bodyweight in relation to the median for the sex/breed, being a terrier breed, being a breed predisposed for parathyroid cancer, being a breed predisposed for thyroid cancer, being a breed predisposed for parathyroid- and/or thyroid cancer, being a breed predisposed for pituitary/adrenal gland cancer, being a breed predisposed for parathyroid- and/or thyroid- and/or pituitary/adrenal gland cancer, being a breed predisposed for mammary gland cancer, being a breed predisposed for testicular/ovarian cancer, being a breed predisposed for reproductive endocrine cancer and being a breed predisposed for one or multiple types of endocrine cancer) and an outcome of being a dog under primary veterinary care in 2019 and having a diagnosis of insulinoma at any date to August 31, 2023. Risk factors with liberal association (P-value < 0.2) in univariable modelling were taken forwards for consideration in multivariable modelling55. Multivariable logistic regression modelling used automated SPSS model-building. Median adult bodyweight is a defining characteristic of individual breeds and therefore was excluded from consideration in initial multivariable breed-focused modelling56. Other variables derived directly from the breed variable (Purebred, Terrier, Breed predisposed for parathyroid cancer, Breed predisposed for thyroid cancer, Breed predisposed for parathyroid- and/or thyroid cancer, Breed predisposed for pituitary/adrenal gland cancer, Breed predisposed for parathyroid- and/or thyroid- and/or pituitary/adrenal gland cancer, Breed predisposed for mammary gland cancer, Breed predisposed for testicular/ovarian cancer, Breed predisposed for reproductive endocrine cancer and Breed predisposed for one or multiple types of endocrine cancer) were similarly not considered in initial breed-focused multivariable modelling. Instead, these variables individually replaced the Breed variable in the final breed-focused multivariable model to evaluate their effects after taking account of the other variables in that model57. The area under the ROC curve was used to evaluate discrimination of the final breed-focused model. Retention in the final multivariable logistic regression model and for statistical significance was set at P < 0.05.
Data availability
The datasets generated during the current study are available at Figshare: dx.doi.org/10.6084/m9.figshare.6025748.
References
-
Steiner, J. M. & Bruyette, D. S. Canine insulinoma. Compend Contin Educ. Pract. Vet. 18, 13–24 (1996).
-
Anderson, C. W. & Bennett, J. Clinical presentation and diagnosis of pancreatic neuroendocrine tumors. Surg. Oncol. Clin. N Am. 25, 363–374 (2016).
-
Starke, A. et al. Malignant metastatic insulinoma—postoperative treatment and follow-up. World J. Surg. 29, 789–793 (2005).
-
Vaidakis, D., Karoubalis, J., Pappa, T., Piaditis, G. & Zografos, G. Pancreatic insulinoma: Current issues and trends. Hepatobiliary Pancreat. Dis. Int. 9, 234–241 (2010).
-
Buishand, F. O., Kik, M. & Kirpensteijn, J. Evaluation of clinico-pathological criteria and the Ki67 index as prognostic indicators in canine insulinoma. Vet. J. 185, 62–67 (2010).
-
Capodanno, Y. et al. Notch pathway inhibition targets chemoresistant insulinoma cancer stem cells. Endocr. Relat. Cancer 25, 131–144 (2018).
-
Capodanno, Y. et al. Canine insulinoma as a model for human malignant insulinoma research: Novel perspectives for translational clinical studies. Transl Oncol. 15, 101269 (2022).
-
Ear, P. H. et al. NET models meeting 2024 White Paper: The current state of neuroendocrine tumour research models and our future aspirations. Endocr. Oncol. Preprint at (2024). https://doi.org/10.1530/EO-24-0055
-
Hofland, J., Refardt, J., Feelders, R. A., Christ, E. & De Herder, W. W. Approach to the patient: Insulinoma. J. Clin. Endocrinol. Metab. 109, 1109–1118 (2024).
-
Ryan, D. et al. Clinical findings, neurological manifestations and survival of dogs with insulinoma: 116 cases (2009-2020). J. Small Anim. Pract. 62, 531–539 (2021).
-
Buishand, F. O. Current trends in diagnosis, treatment and prognosis of canine insulinoma. Vet. Sci. 9, 540 (2022).
-
Thakker, R. Multiple endocrine neoplasia—syndromes of the twentieth century. J. Clin. Endocrinol. Metab. 83, 2617–2620 (1998).
-
Beatrice, L. et al. Concurrent endocrine neoplasias in dogs and cats: A retrospective study (2004–2014). Vet. Rec 182, 323 (2018).
-
Khanna, L. et al. Pancreatic neuroendocrine neoplasms: 2020 update on pathologic and imaging findings and classification. Radiographics 40, 1240–1262 (2020).
-
Hassan, M. M. et al. Family history of cancer and associated risk of developing neuroendocrine tumors: A case-control study. Cancer Epidemiol. Biomarkers Prev. 17, 959–965 (2008).
-
Capurso, G. et al. Risk factors for sporadic pancreatic endocrine tumors. Am. J. Gastroenterol. 104, 3034–3041 (2009).
-
Halfdanarson, T. R. et al. Risk factors for pancreatic neuroendocrine tumors: A clinic-based case-control study. Pancreas 43, 1219–1222 (2014).
-
Ben, Q. et al. Risk factors for sporadic pancreatic neuroendocrine tumors: A case-control study. Sci. Rep. 6, 36073 (2016).
-
Valente, R. et al. Risk and protective factors for the occurrence of sporadic pancreatic endocrine neoplasms. Endocr. Relat. Cancer 24, 405–414 (2017).
-
Zhan, H., Liu, C., Zhao, Y., Zhang, T. & Chen, G. Risk factors for the occurrence of insulinoma: A case-control study. Hepatobiliary Pancreat. Dis. Int. 12, 324–328 (2013).
-
Polton, G., White, R. N., Brearley, M. J. & Eastwood, J. M. Improved survival in a retrospective cohort of 28 dogs with insulinoma. J. Small Anim. Pract. 48, 151–156 (2006).
-
Del Busto, I. et al. Incidence of postoperative complications and outcome of 48 dogs undergoing surgical management of insulinoma. J. Vet. Intern. Med. 34, 1135–1143 (2020).
-
Cleland, N. T., Morton, J. M. & Delisser, P. J. Outcome after surgical management of canine insulinoma in 49 cases. Vet. Comp. Oncol. 19, 428–441 (2020).
-
Coss, P., Gilman, O., Warren-Smith, C. & Major, A. C. The appearance of canine insulinoma on dual phase computed tomographic angiography. J. Small Anim. Pract. 62, 540–546 (2021).
-
Petrelli, A., German, A. J., O’Connell, E. M. & Silvestrini, P. Serum insulin concentration in dogs with insulinoma as a clinical marker for presence of metastasis at the time of diagnosis. J. Vet. Intern. Med. 37, 1139–1145 (2023).
-
Taruscio, D., Vittozzi, L., Rocchetti, A., Torreri, P. & Ferrari, L. The occurrence of 275 rare diseases and 47 rare disease groups in Italy. Results from the national registry of rare diseases. Int. J. Environ. Res. Public. Health 15, 1470 (2018).
-
Major, A., Cox, S. M. & Volchenboum, S. L. Using big data in pediatric oncology: current applications and future directions. Semin Oncol. 47, 56–64 (2020).
-
VetCompass. VetCompass programme. (2024). http://www.rvc.ac.uk/VetCOMPASS/.
-
Service, F. J., McMahon, M. M., O’Brien, P. C. & Ballard, D. J. Functioning insulinoma—incidence, recurrence, and long-term survival of patients: A 60-year study. Mayo Clin. Proc. 66, 711–719 (1991).
-
Halfdanarson, T. R., Rabe, K. G., Rubin, J. & Petersen, G. M. Pancreatic neuroendocrine tumors (PNETs): Incidence, prognosis and recent trend toward improved survival. Ann. Oncol. 19, 1727–1733 (2008).
-
Sada, A. et al. Malignant insulinoma: A rare form of neuroendocrine tumor. World J. Surg. 44, 2288–2294 (2020).
-
Nockel, P. et al. Localization of insulinoma using 68Ga-DOTATATE PET/CT scan. J. Clin. Endocrinol. Metab. 102, 195–199 (2017).
-
O’Neill, D. G. et al. French Bulldogs differ to other dogs in the UK in propensity for many common disorders: A VetCompass study. Canine Med. Genet. 8, 13 (2021).
-
Cohen, D., Reif, J. S., Brodey, R. S. & Keiser, H. Epidemiological analysis of the most prevalent sites and types of canine neoplasia observed in a veterinary hospital. Cancer Res. 34, 2859–2868 (1974).
-
Shoop, S. J. W. et al. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet. Epidemiol. 2, 1 (2015).
-
Pittaway, C., Schofield, I., Dobson, J. E., O’Neill, D. G. & Brodbelt, D. Incidence and risk factors for the diagnosis of lymphoma in dogs in UK primary-care practice. J. Small Anim. Pract. 60, 581–588 (2019).
-
Edmunds, G. et al. Dog breeds and body conformations with predisposition to osteosarcoma in the UK: A case-control study. Canine Med. Genet. 8, 2 (2021).
-
Aupperle-Lellbach, H. et al. Tumour incidence in dogs in Germany: A retrospective analysis of 109,616 histopathological diagnoses (2014–2019). J. Comp. Pathol. 198, 33–55 (2022).
-
Śmiech, A., Bulak, K., Łopuszyński, W. & Puła, A. Incidence and the risk of occurrence of benign and malignant canine skin tumours in Poland – a five-year retrospective study. J. Vet. Res. 67, 437–446 (2023).
-
Parker, H. G. et al. Genomic analyses reveal the influence of geographic origin, migration, and hybridization on modern dog breed development. Cell. Rep. 19, 697–708 (2017).
-
De León-Nava, M. A. et al. Immune sexual dimorphism: Effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J. Steroid Biochem. Mol. Biol. 113, 57–64 (2009).
-
Goutal, C. M., Brugmann, B. L. & Ryan, K. A. Insulinoma in dogs: A review. J. Am. Anim. Hosp. Assoc. 48, 151–163 (2012).
-
Lauby-Secretan, B. et al. Body fatness and cancer—viewpoint of the IARC working group. N Engl. J. Med. 375, 794–798 (2016).
-
Chandler, M. et al. Obesity and associated comorbidities in people and companion animals: A one health perspective. J. Comp. Pathol. 156, 296–309 (2017).
-
Pegram, C. et al. Frequency, breed predisposition and demographic risk factors for overweight status in dogs in the UK. J. Small Anim. Pract. 62, 521–530 (2021).
-
Kiupel, M., Mueller, P. B., Vara, J., Irizarry, A. R. & Lin, T. L. Multiple endocrine neoplasia in a dog. J. Comp. Pathol. 123, 210–217 (2000).
-
Marini, F., Gisuti, F. & Brandi, M. L. Genetic disorders and insulinoma/glucagonoma. Endocr. Relat. Cancer 31, e230245 (2024).
-
O’Neill, D. G., Church, D., McGreevy, P. & Thomson, P. Brodbelt, D. Approaches to canine health surveillance. Canine Genet. Epidemiol. 1, 2 (2014).
-
Streiner, D. L. & Norman, G. R. Correction for multiple testing: Is there a resolution? Chest 140, 16–18 (2011).
-
Asher, L. et al. Estimation of the number and demographics of companion dogs in the UK. BMC Vet. Res. 7, 74 (2011).
-
Centers for Disease Control and Prevention. Epi Info. (2022). https://www.cdc.gov/epiinfo/index.html
-
The VeNom Coding Group. VeNom Veterinary Nomenclature. http://venomcoding.org/ (2022).
-
The Kennel Club. Breed Information Centre: Breeds A to Z. (2024). https://www.thekennelclub.org.uk/search/breeds-a-to-z
-
Scott, M., Flaherty, D. & Currall, J. Statistics: How many? J. Small Anim. Pract. 53, 372–376 (2012).
-
Dohoo, I., Maritin, W. & Stryhn, H. Model-building strategies in Veterinary Epidemiologic Research (ed McPike, M.) 370–371 (VER Inc, 2009).
-
The Kennel Club. Breed standards: The Kennel Club (2024). https://www.thekennelclub.org.uk/breed-standards
-
O’Neill, D. G., Rowe, D., Brodbelt, D. C., Pegram, C. & Hendricks, A. Ironing out the wrinkles and folds in the epidemiology of skin fold dermatitis in dog breeds in the UK. Sci. Rep. 12, 10553 (2022).
Acknowledgements
Thanks to Noel Kennedy (RVC) for VetCompass™ software and programming development. We acknowledge the Medivet Veterinary Partnership, Vets4Pets/Companion Care, Goddard Veterinary Group, CVS Group, IVC Evidensia, Linnaeus Group, Beaumont Sainsbury Animal Hospital, Blue Cross, PDSA, Dogs Trust, Vets Now and the other UK practices who collaborate in VetCompass™. We are grateful to The Kennel Club Charitable Trust, Agria Pet Insurance and The Kennel Club for supporting VetCompass.
Funding
This study was supported at the RVC by an award from the Kennel Club Charitable Trust and Agria Pet Insurance. Neither the Kennel Club Charitable Trust, Agria Pet Insurance nor the Kennel Club had any input in the design of the study, the collection, analysis and interpretation of data or in writing the manuscript. LJD. has been supported by an MRC Clinician Scientist Fellowship (MR/R007977/1) and is currently supported by an MRC Transition Support Award (MR/X023559/1).
Author information
Authors and Affiliations
Contributions
DON and DCB were responsible for the acquisition of the clinical data used in the study. KK, DON and FOB were responsible for the conception and design. KK and DON were responsible for the extraction and collation of data. KK carried out the analysis. KK, DON and FOB were mainly responsible for drafting the manuscript. KK, DON, FOB, DCB, LJD and SG were involved in interpreting the results, revising the manuscript and gave final approval of the version to be published. KK, DON, FOB, DCB, LJD and SG agree to be accountable for all aspects of the accuracy and integrity of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kraai, K., O’Neill, D.G., Davison, L.J. et al. Incidence and risk factors for insulinoma diagnosed in dogs under primary veterinary care in the UK.
Sci Rep 15, 2463 (2025). https://doi.org/10.1038/s41598-025-86782-6
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-86782-6
Keywords
This post was originally published on this site be sure to check out more of their content.