What’s New
The Food and Drug Administration’s (FDA) Center for Veterinary Medicine posted a letter to veterinarians on Monday alerting them of adverse effects reported in dogs following injections of Librela, a treatment aimed at providing pain relief for canines with osteoarthritis.
Why It Matters
Dogster reports that there are an estimated 86.9 million dogs in the U.S. The Morris Animal Foundation said about 14 million of those dogs live with osteoarthritis, also known as degenerative joint disease, which is a “progressive disease of the joints fueled by chronic inflammation. The end result is chronic pain that can have far-ranging, negative health effects beyond an affected joint.”
What Are the Side Effects of Librela in Dogs?
The FDA warned of several neurological and physical problems in dogs after receiving Librela injections.
The letter comes after several reports revealed the adverse effects of the drug.
Zoetis, manufacturer of Librela, shared the company’s statement with Newsweek via email: “The information shared by the FDA is consistent with what we’ve seen in our pharmacovigilance data. We maintain regular communication with the FDA to ensure the ongoing safety and efficacy of our products.”
The letter details several side effects, including ataxia—which causes lack of muscle control and coordination, seizures, paresis, recumbency, urinary incontinence, polyuria, and polydipsia.
The most intense side effect is death, both naturally and by euthanasia.
Users on a Facebook group, Librela: The REAL Truth in Testimony, often post about side effects they’ve noticed in their dogs who have been treated with the injection, some noticing spasms, neurological problems, and lack of appetite, among other effects.
What To Know
Librela is a monoclonal antibody drug that was approved by the FDA on May 5, 2023. Veterinarians administer the drug as a once-a-month injection, with the dosage determined by the dog’s weight. It is available as a 5, 10, 15, 20, or 30 mg/ml solution injection.
Zoetis’ statement notes that the FDA’s action is not a “warning,” but is a “‘Dear Vet’ letter which serves as a safety update.”

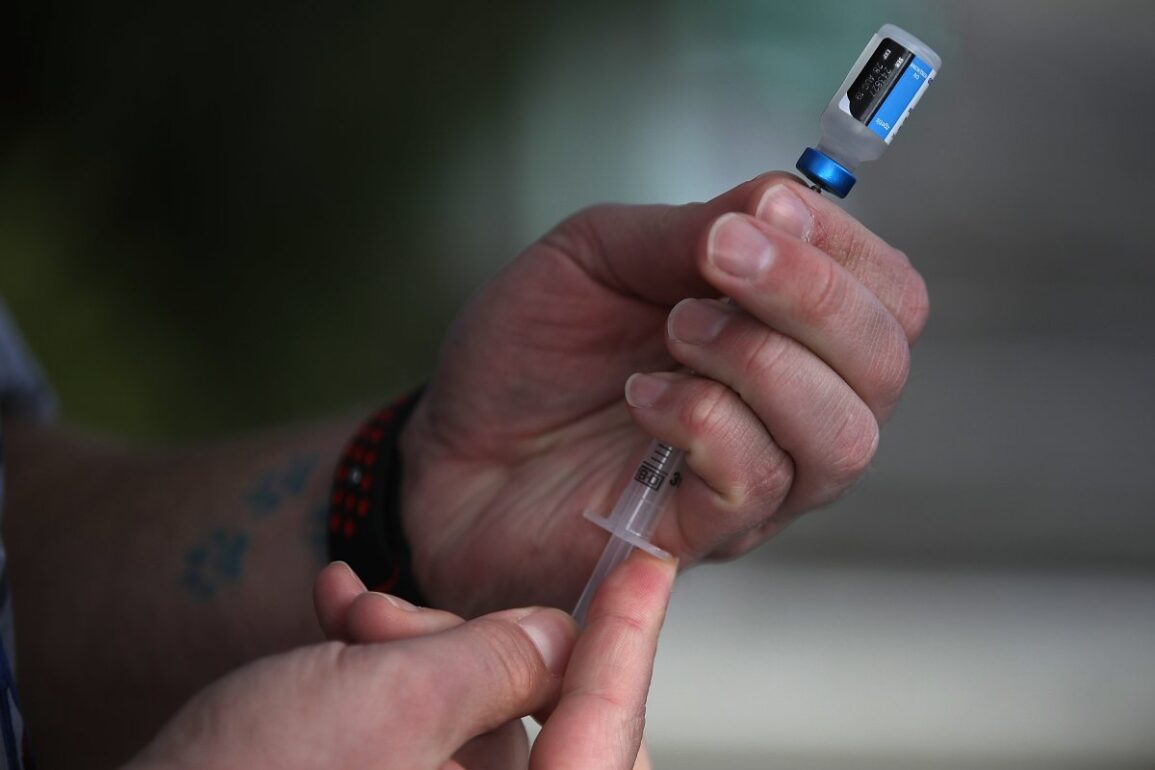
Veterinarian technician Justin Jones prepares a syringe with a canine influenza vaccine at Los Gatos Dog and Cat Hospital on January 25, 2018, in Los Gatos, California. The FDA on Monday notified veterinarians about adverse effects in dogs resulting from injectable drug Librela.
Justin Sullivan/Getty Images
How Successful Is Librela for Dogs?
Librela works by blocking the pain pathway from the joint to the brain, reducing the signals sent, therefore providing dogs with some relief from pain.
A U.S. study of 272 dogs with osteoarthritis found that 47.4 percent of dogs receiving the drug showed improvement after the first shot, while 36.6 percent of dogs receiving the placebo noted improvement. The study was published in 2023.
Zoetis’ statement noted that more than 21 million doses of the drug have been distributed globally.
How Much Does Librela Cost for Dogs?
The cost of Librela varies based on the amount needed for the dog, with estimates ranging from $60 to $150 per injection. Additional fees may apply for veterinarian consultation and administration.
What Happens Next
The letter informed veterinarians to report any adverse effects to Zoetis at 1-888‑963-8471, along with the dog’s medical history, “how many times the dog has received Librela, and the lot number on the vial used.” Drug sponsors are required to inform the FDA of any reports of adverse effects, both from pet owners and veterinarians.
The FDA can encourage animal drug manufacturers to update labels with additional information regarding shown adverse effects. “Zoetis is in discussions with the FDA on label updates and expects it will reflect post approval adverse event reporting and be similar to labels in other markets,” the company’s statement said.
The agency also suggests veterinarians review several of the recent reports that detail the drug’s side effects, and noted that if any additional information comes to light, it will be made available.
Update 12/18/24, 4:16 p.m. ET: This article has been updated to include Zoetis’ statement.
Correction 12/18/24, 4:50 p.m. ET: This article and headline have been changed to reflect that the FDA issued a letter to veterinarians, not a warning, over the drug Librela.
This post was originally published on this site be sure to check out more of their content.