Abstract
Dogs serve as a promising aging model due to their genetic diversity, condensed lifespan, and shared living environment with humans. Alterations in the immune and metabolic parameters are hallmarks of aging in humans, but few studies have investigated these changes in dogs. We investigated the association of whole blood parameters with aging in a cross-sectional field study with a population of 451 companion dogs. Additionally, we measured total lymphocytes, total T-cells, CD4 T-cells, CD8 T-cells, B-cells, CBC, insulin and adiponectin in a cross-sectional study of 74 laboratory research beagles. In companion dogs, we report total lymphocytes and RBCs decrease significantly with age while platelets increase significantly. In lab beagles, total lymphocytes, T-cells, CD4 T-cells, CD8 T-cells, and B cells are significantly lower in Aged and Geriatric beagles. Furthermore, the CD4/CD8 ratio is significantly lower in Geriatric beagles. We also found that Geriatric beagles experience hyperinsulinemia, while plasma adiponectin is significantly lower in both Aged and Geriatric beagles. These results align with the age-related immune and metabolic alterations seen in humans and provide additional evidence that dogs serve as a relevant translational model of aging.
Introduction
Aging is a highly complex, multifactorial process that differentially affects all organ systems. Due to the time required to study physiological and pathological changes that occur with aging in human populations, model species that reflect these changes in a condensed time frame are of critical need. Challenges of using rodent and invertebrate models include limited genetic and environmental diversity, as well as distance from humans on the phylogenetic tree1. Dogs provide a unique opportunity to address these challenges because they have condensed lifespans compared to humans2. Similarly to humans, dogs vary greatly in size and genetics, variables shown to affect their predisposition to certain diseases and influence lifespan3. Importantly, companion dogs share a similar living environment to humans, unlike commonly used preclinical species (e.g. rodents) that are studied in artificially controlled laboratory environments. These factors may play a role in both humans and dogs developing similar age-related diseases, such as osteoarthritis, sarcopenia and cancer2,4,5.
The characteristics and composition of blood change as an element of the natural aging process. These characteristics, including cell count, size, shape and surface protein (marker) expression, have all been shown to change with age in humans6. The Complete Blood Count (CBC) test, a frequently used clinical diagnostic tool and evaluation of general health, measures cell counts and certain cell properties in blood samples. Investigations into the age-related changes in CBC reported that the counts of lymphocytes, red blood cells (RBCs), and platelets are shown to decrease with age while neutrophil count increases7,8,9. Similarly in dogs, an investigation into a heterogeneous population of companion dogs found that lymphocyte counts were negatively associated with age, but no significant age-associated changes for RBCs or platelets were reported10.
Age-related changes in the immune system and immune function have also been extensively described in humans. The thymus, an immune organ that is critical for lymphocyte maturation, has been shown to atrophy with advanced age in humans11. This thymic atrophy leads to reduced lymphocyte production and disrupts the relative balance of distinct lymphocyte subsets, which can compromise the adaptive immune response. To this end, the total number of lymphocytes, as well as the absolute counts of individual lymphocyte subsets, have been reported to decline with age. Additionally, total lymphocytes, T-cells (CD3+), helper T-cells (CD3 + CD4+), cytotoxic T-cells (CD3 + CD8+), and B-cells (CD3-CD21+) have all been shown to decrease as humans age11,12,13,14. Furthermore, the ratio of CD4 + T-cells to CD8 + T-cells (CD4/CD8) has also been observed to decline12. Indeed, low lymphocyte levels and a reduced CD4/CD8 ratio are associated with impairment of the adaptive immune system, which leads to functional consequences, such as poor vaccine efficacy and increased susceptibility to infections and cancer15,16.
These changes in lymphocyte populations have also been described in dogs. Decreases in total lymphocytes, T-cells, CD4 T-cells, and CD8 T-cells with age have been reported in a longitudinal study of Labrador retriever dogs17. In a separate cross-sectional study, lymphocyte populations in older dogs were seen to have lower percentages of B-cells and higher percentages of total T-cells and CD8 T-cells, while the percentage of CD4 T-cells was unchanged18. Recently, a cross-sectional pilot study in a mixed population of companion animals demonstrated that total lymphocyte counts decreased with age, however lymphocyte subpopulations were not examined10. While these trends point to similarities in age-related immune cell fluctuations in dogs and humans, there are scant dog studies available in the literature, none of which quantify lymphocyte subsets in a genetically diverse population of dogs.
In addition to age-related immune cell and function changes, fasting metabolic parameters have also been described to change with age across several species. Notably, lipid markers of metabolism, such as cholesterol and free fatty acids, were reported to increase with age and associate with increased frailty in dogs19. Two hormones in particular, adiponectin and insulin, have been extensively studied due to their pleiotropic effects on insulin sensitivity and glucose homeostasis in aging mammals20,21. Fasting insulin and indices that account for fasting insulin (e.g. HOMA-IR) have been used to assess the presence of hyperinsulinemic compensation and insulin resistance in human populations with aging-related diseases22. In humans and rodents, insulin clearance deteriorates with age, which associates with a broader decline in overall insulin sensitivity23. Similarly in dogs, it has been reported that dogs experience age-related hyperinsulinemia and peripheral insulin resistance24.
One important factor that could influence age-related changes in immune function is adiponectin, which is an adipokine that has been described to have insulin sensitizing effects in metabolic tissues (e.g. muscle, liver), as well as anti-inflammatory effects in immune cell populations (e.g. macrophages)25,26. In humans, adiponectin concentrations are reported to decrease with development and progression of several age-related diseases25. Conversely, populations of long lived humans (e.g. centenarians) have been described to exhibit high levels of circulating adiponectin27. While the effects of adiponectin are well characterized in humans and rodents, the associations of low adiponectin with diseases has only been sparsely investigated in dogs, with lower levels of adiponectin seen in certain disease states such as sepsis and mitral valve disease compared to healthy dogs28,29.
Collectively, there are relatively few studies examining alterations in these immune and metabolic parameters in aging dogs. Therefore an assessment of whether these pathobiological alterations are conserved in dogs is integral in determining their potential as a model species to study aging. In this observational study, we aimed to assess differences in total lymphocytes, RBCs and platelets in a cross sectional cohort of 451 real-world, companion dogs previously described by Chen et al., 202330. In addition, we aimed to cross-sectionally characterize immune and metabolic changes in a population of young, aged and geriatric laboratory beagles by measuring CBC, lymphocyte subsets, insulin and adiponectin.
Methods
Companion dog field study design
Study design, methods, population demographics, dog breeds and clinical chemistry have been described previously19,30. In brief, this investigation was performed according to VICH-GL9 Good Clinical Practice (GCP) standards and approved by an independent IACUC through Veterinary and Biomedical Research Center, Inc. (VBRC) (Approval no.: VACLOY001CLDEFF-1PILC). All investigators and study veterinarians consisted of licensed veterinarians. Owner consent was obtained prior to initiating study procedures. This study is reported in accordance with ARRIVE guidelines.
Eligibility criteria for the study included dogs from ages ≥ 2 and < 6 years (‘Young’) or ≥ 7 years (‘Old’) and if body weight was < 11.3 kg (25 lbs) (small) or > 22.7 kg (50 lbs) (large). The study enrolled 451 eligible (406 with complete data), mature, adult dogs consisting of 43.6% mixed breeds and 56.4% purebred dogs. The mean age was 7.72 years (range = 2.0 to 17.7, SD = 3.8) and the mean weight was 24.0 kg (range = 1.3 to 80, SD = 15).
Fasted blood samples were collected in veterinary clinics by venipuncture in a serum separator tube. The protocol required at least 6 h of fasting. However, fasting adherence was not confirmed. Samples were settled at room temperature for ≥ 60 min prior to centrifugation at 2800–3200 rpm for 10 min at ambient temperature. Resulting serum was divided into aliquots of approximately 500–1000µL using 1.8 mL cryovials and stored at approximately − 80 °C until shipment to be analyzed by IDEXX Bioanalytics (Sacramento, CA).
Laboratory beagle study design
Seventy-four adult male and female laboratory beagles weighing 8.3 to 14.9 kg were included in the study. The dogs were stratified into three groups determined by their age. The “Young” group consisted of 40 beagles ranging from 1.4 to 4.7 years old, the “Aged” group consisted of 21 beagles ranging from 8.0 to 9.1 years old, and the “Geriatric” group consisted of 12 beagles ranging from 10.5 to 13.5 years old. No dogs were excluded on the basis of age; gaps in the age range between groups (e.g. 5 to 6 years old) are due to limited age diversity present in the cohort. This study is reported in accordance with ARRIVE guidelines.
Blood collections for endpoint analysis were completed at a single time point, across four separate groups of animals. Protocols were approved by the institutional animal care and use committee (IVS218-22027-DZ, IVS218-22045-DZ).
Following a 14 h fast, blood was collected via jugular venipuncture to assess insulin, adiponectin, flow cytometry and CBC. Whole blood was transferred into a K2EDTA tube and gently inverted. Samples were centrifuged at 2800–3200 RPM for 10 min at 4 °C. Resulting plasma was frozen at -80 °C until analysis.
Insulin, adiponectin, and CBC
Circulating canine insulin and total adiponectin concentrations were measured from plasma by single-analyte ELISA (Canine Insulin ELISA, Mercodia 10-1203-01; CircuLex Dog Adiponectin ELISA, MBL International CY-8052, respectively) and analyzed by Mercodia AB (Sylveniusgatan 8 A, SE-754 50 Uppsala, Sweden). CBC analysis was completed from fresh whole blood by IDEXX Bioanalytics (Sacramento, CA).
Antibodies
Lymphocyte markers were selected based on a clinically validated panel for the diagnosis of leukemia in dogs31. CD3 is a marker of mature T-cells, CD4 is a marker of helper T-cells, CD8 is a marker of cytotoxic T-cells, and CD21 is a marker of mature B-cells. FITC-conjugated anti-CD3 (CA17.2A12), PE-conjugated anti-CD21 (CA2.1D6), Pacific Blue-conjugated anti-CD4 (YKIX302.9), and AF647-conjugated anti-CD8 (YCATE55.9) were sourced from BioRad (Hercules, CA).
Flow cytometry
Whole blood was stained 1:10 with each antibody. Samples were mixed by pipetting and incubated 15 min in the dark at room temperature. Samples were then lysed with 1X PharmLyse (BD Biosciences), mixed by pipetting and incubated for 10 min in the dark at room temperature. After, samples were centrifuged at 1700 rpm for 5 min at room temperature with brake. Supernatant was then discarded, and samples were washed once with 1X DPBS CMF (Life Technologies). After washing, samples were resuspended in 125 µl of 1X DPBS CMF.
Stained cells were acquired on a FACSCanto™ flow cytometer and analyzed using BD FACSDiva™ software version 8.0 (BD Biosciences). The flow cytometer was set to collect 20,000 lymphocyte events in each tube. The lymphocyte population was determined by electronic gating on the basis of forward versus side scatter. Subpopulations were identified by fluorescent antibodies for specific cell markers (Fig. S1). Sample analysis was performed at Translational Drug Development (Bothell, WA).
Statistical analysis
One female aged beagle was removed for an outlier insulin value that was below the limit of quantification. One female geriatric beagle was removed for an outlier CD4/CD8 value that was 2.9, which was greater than two standard deviations of the mean. One female geriatric beagle was removed because it developed dermatitis and received treatment with an immunosuppressant. If platelet clumping was observed in the platelet sample analysis, that beagle was removed from the platelet analysis.
The laboratory beagle cohort was divided into three age groups, in parallel with the study design, defined as: “Young” (1-4.99 years), “Aged” (8-9.99 years), and “Geriatric” (10 + years). Additionally, there was limited variability in continuous age within age groups of the laboratory beagles, precluding the ability to treat age as a continuous variable within or across groups.
In the companion dog cohort, age was assessed as both a continuous and a categorical variable to facilitate comparisons with the laboratory beagle cohort. Age groups were defined as: Young” (2-5.99 years), “Aged” (7-9.99 years), and “Geriatric” (10 + years).
Demographics of the laboratory beagle cohort (age, weight and sex) as well as endpoints are summarized using mean, standard error, and ranges stratified by age groups and for the groups overall. Detailed demographics of the companion dog cohort are previously reported in Chen et al., 202330. A reduced set of demographics of the companion dog cohort were described here.
Associations between age groups and individual markers were tested using multiple regression, with age as the primary predictor. Overall effects of age were tested using F-test. In the beagle cohort, body weight (kg) was the only available candidate covariate and was individually added to each model. If the addition of body weight significantly improved model fit (F-test resulting in p < 0.05), it was included in the model as a covariate. For the companion dog cohort, body condition score, neuter status, and sex were also considered as possible covariates under the same methodology. Only covariates which reached significance were included in the models and reported in their respective tables.
Post-hoc pairwise comparisons of differences in markers across age groups were performed, using Tukey adjustment for multiple comparisons. Assumptions of normality and homoscedasticity were assessed using visual inspection (histogram and quantile-quantile plots) and Levene’s test for equal variances, respectively.
To leverage the breadth of age ranges captured in the companion dog cohort, age was also tested as a continuous measure. As the effect of age on individual markers may change over the lifespan, an interaction between continuous age and age group (defined above) was included, allowing for estimates of rates of change in young, aged, and geriatric dogs separately. Estimated marginal means of linear trends (e.g., slopes for each age group) were estimated from these models to estimate aging-related changes in marker levels across these age groups. A comparable analysis could not be performed in the laboratory beagles due to limited variability in age ranges in the cohort.
All tests were tested with a two-sided alpha = 0.05. All statistics were performed using R version 4.3.2.
Results
Companion dog study demographics
The cross-sectional companion dog study (Healthspan) included a total of 451 dogs of which 46.3% were female and 53.7% were male. The mean age of dogs was 7.72 years (range = 2.0 to 17.7, SE = 0.2). Mean weight was 24.0 kg (range = 1.3 to 80, SE = 0.7) and mean Body Condition Score was 5.4 (range = 3 to 0, SE = 0.0) (Table 1). The majority (91.1%) of dogs were neutered. The population consisted of 43.6% mixed breeds and 56.4% purebred dogs. Full demographics from this population are reported by Chen et al., 202330.
Laboratory beagle cohort demographics
The cross-sectional laboratory beagle study included a total of 74 beagles of which 59.5% were female and 40.5% were male (Table 1). The mean age of dogs was 6.63 years (range = 1.4 to 13.5, SE = 0.39). Mean weight was 11.13 kg (range = 8.3 to 14.9, SE = 0.18). All dogs were intact, purebred beagles with no underlying health issues.
The dogs were stratified into three groups determined by their age range. The “Young” group consisted of 40 beagles ranging from 1.4 to 4.7 years old, the “Aged” group consisted of 21 beagles ranging from 8.0 to 9.1 years old, and the “Geriatric” group consisted of 12 beagles ranging from 10.5 to 13.5 years old. Beagles were binned into three age groups as opposed to using a linear regression method because age sampling diversity was low.
CBC in companion dogs
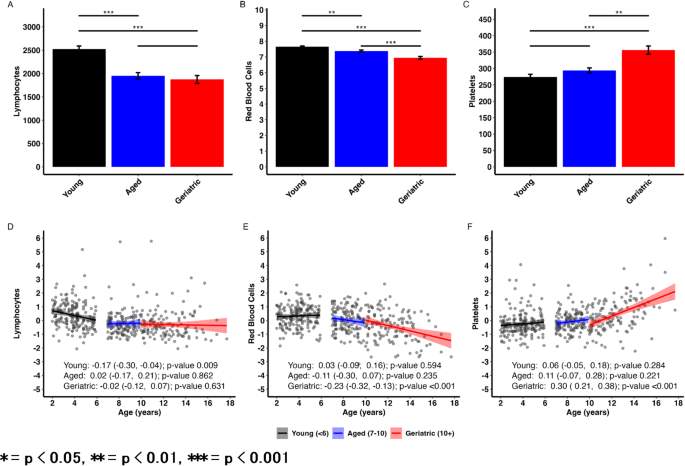
CBC results from companion dogs can be found in Tables 2 and 3, and Fig. 1. Lymphocytes and RBC counts both significantly declined in Aged and Geriatric dogs compared to Young dogs (p < 0.01, Fig. 1A-B). While there was no significant difference between Aged and Geriatric lymphocyte counts, RBC counts were decreased in geriatric dogs compared to aged dogs (p < 0.001, Fig. 1B). Platelets significantly increased in Geriatric dogs compared to Young dogs (p < 0.001, Fig. 1C) and Aged dogs (p < 0.001). However, there were no significant differences between platelet counts between Aged and Young dogs.
Lymphocytes and red blood cells decline with age while platelets increase with age in companion dogs. (A-C) CBC parameter results from Young, Aged, and Geriatric dogs from the companion dog cohort. All values reported as mean ± SEM. P-values measured via pairwise comparisons of multiple regression with age group as main effect and body weight and condition score as covariates. Tukey adjustments for multiple comparisons were used. (D-F) Scatter plots showing associations between age and CBC parameters stratified by age group. Plot text reflects estimated marginal trends (i.e. slope estimates) from a model with age by age group interaction and body weight and condition score as covariates.
Scatter plots and estimated marginal trends for age can be found in Fig. 1D-F. Outcomes are standardized (mean = 0, SD = 1) to facilitate comparison of effect sizes across parameters. Age-related changes in lymphocytes are the most dramatic in Young dogs (Slope = -0.17, p = 0.009) where changes begin to flatten once dogs reach 7 + years of age. RBCs showed an accelerating decline with age where Geriatric dogs showed significant age-related declines (Slope = -0.23, p < 0.001). Platelet count showed accelerating increase with age, with Geriatric dogs showing significant age-related increases (Slope = 0.30, p < 0.001). As all outcomes were normalized, these slopes can be interpreted as 1 year increase in age is associated with a [slope] standard deviation change in a given parameter.
CBC in laboratory Beagles
CBC results from laboratory beagles are summarized in Tables 4 and 5, and Fig. 2. Similar to what was observed in companion dogs, RBC count significantly declined in Aged and Geriatric beagles compared to Young beagles (p < 0.001), however, there was no difference detected between Aged vs. Geriatric groups (Fig. 2A-C). Similarly, hematocrit was significantly lower in the Aged and Geriatric groups compared to Young (p < 0.01), but there was no difference between Aged vs. Geriatric. Hemoglobin followed the same trend, where levels in Aged and Geriatric dogs were significantly lower than Young beagles (p < 0.01; p < 0.001, respectively) while Aged vs. Geriatric comparison showed no significant difference. Platelet count was significantly higher in Geriatric beagles compared to Young and Aged beagles (p < 0.001), resembling the relationship seen in companion dogs (Fig. 2D). While Geriatric beagles had greater platelet counts than Young and Aged dogs, there was no significant difference detected between the Young vs. Aged beagles.
Red blood cells, hematocrit and hemoglobin levels are reduced in Aged and Geriatric beagles while platelet count is greater in Geriatric laboratory beagles. Hematology test results from Young, Aged, and Geriatric beagles. (A) Red blood cell count, (B) hematocrit, and (C) hemoglobin and (D) platelet count in Young beagles (n = 30) compared to Aged (n = 17) and Geriatric (n = 11) beagles. All values reported as mean ± SEM. P-values measured via pairwise comparisons of One-Way ANOVA with age group as main effect.
Lymphocyte populations in laboratory Beagles
The results for total lymphocytes and lymphocyte subsets are summarized in Table 2; Fig. 3. In addition, a representative flow cytometric analysis used to acquire lymphocyte subset data is shown in Supplemental Fig. 1. Similarly to what was shown in the companion dog cohort, Aged and Geriatric beagles had significantly lower total lymphocyte counts than Young beagles (p < 0.001). The mean lymphocyte count in Geriatric beagles was lower than Aged beagles, however the difference was not statistically significant (Fig. 3A).
Total lymphocytes and lymphocyte subpopulations are reduced in Aged and Geriatric laboratory beagles. Cell counts in Young, Aged, and Geriatric beagles measured by flow cytometry. (A) Lymphocytes, (B) CD3+, (C) CD3-CD21+, (D) CD3 + CD4 + and (E) CD3 + CD8 + counts and (F) CD4/CD8 ratio in Young beagles (n = 30) compared to Aged (n = 17) and Geriatric (n = 11) beagles. All values reported as mean ± SEM. P-values measured via pairwise comparisons of One-Way ANOVA with age group as main effect.
Similarly, Aged and Geriatric beagles had significantly lower CD3 + counts than Young beagles (p < 0.001). CD3 + count in Geriatric dogs was lower than that of Aged dogs, but the difference was not significant (Fig. 3B). CD3- CD21 + cell count was significantly lower in Aged and Geriatric dogs compared to Young dogs (p < 0.05; p < 0.001, respectively). However, the CD3- CD21 + cell count reduction in Geriatric vs. Aged was not statistically significant (Fig. 3C).
Aged and Geriatric beagles had significantly lower CD3 + CD4 + cell counts than Young beagles (p < 0.001). CD3 + CD4 + cell count in Geriatric beagles was lower than Aged beagles, however the difference was also not statistically significant (Fig. 3D). CD3 + CD8 + cell counts were significantly lower in the Aged and Geriatric groups compared to the Young group as well (p < 0.05). There was no significant difference in CD3 + CD8 + count for Geriatric vs. Aged comparison (Fig. 3E). The ratio of CD4 + cells to CD8 + cells (CD4/CD8) trended towards reduction but was only significantly lower in the Geriatric vs. Young group comparison (p < 0.05, Fig. 3F).
Insulin and adiponectin in laboratory Beagles
The results for plasma insulin and adiponectin levels are summarized in Table 2; Fig. 4. Fasting insulin levels were significantly elevated in Geriatric dogs compared to Aged dogs (p < 0.05; Fig. 4A). Adiponectin concentrations were significantly higher in Young dogs compared to both Aged and Geriatric dogs (p < 0.05; Fig. 4B).
Insulin concentrations are higher in Geriatric laboratory beagles while adiponectin is lower in Aged and Geriatric beagles. (A) Fasting insulin and (B) adiponectin concentrations in Young beagles (n = 40) compared to Aged (n = 21) and Geriatric (n = 13) beagles. All values reported as mean ± SEM. P-values measured via pairwise comparisons of One-Way ANOVA with age group as main effect.
Discussion
The changes in immune and metabolic function are central elements of mammalian aging and age-related diseases. These changes are most well-described in humans and preclinical models, such as rodents, that have been used as a model to study age-related disease32. Investigations into real world populations of companion dogs have gained popularity due to their exposure to similar environmental factors, natural development of similar age-related diseases and potential for greater translatable capacity. In addition, laboratory dogs may experience relevant age-related changes, similar to those seen in humans and real-world companion dogs. In this study we aimed to examine the changes in immune populations in both real-world companion dogs and a cohort of laboratory beagles of varying age to determine if changes in immune populations were conserved across species. Additionally in the laboratory beagles we measured insulin and adiponectin, two key metabolic hormones that are integral to age-related disease.
Consistent with findings in humans, we observed that lymphocyte count declines with age in both companion dogs and laboratory beagles7. Our data recapitulate a recent field study in companion animals that found the same result10. Expanding on these findings, we also found that RBC decreases with age in companion dogs, and RBC, hematocrit and hemoglobin levels decrease with age in laboratory beagles, mirroring age-related declines seen in humans with advanced age9.
In humans, platelet count has been reported to decrease with age due to the gradual loss of hematopoietic tissue in the bone marrow33. However an inversion of this relationship may be related to certain aging-related diseases such as sarcopenia34. Several investigations have reported that elevations in platelet count are independently associated with muscle loss and weakness from sarcopenia35. Both our investigation into companion dogs and laboratory beagles found that platelet count increases with age. This suggests that aging dogs capture an element of aging-related disease that is infrequently observed in healthy, aging humans. Of note, a field study in companion dogs reported a weak positive correlation between age and platelet count that was lost with multiple comparisons10. Differences in the sampled population and cohort size may explain the loss of correlation with multiple comparisons seen in the field study. More investigations are warranted investigating the relationship between age-related changes in platelet count and muscle loss and weakness in dogs.
Although CBC can reveal changes in total lymphocyte count, it is unable to differentiate between lymphocyte subpopulations such as T-cells and B-cells. Changes in T-cell and B-cell counts are indicative of immune system dysfunction, and they have also been reported in aging humans36. We aimed to assess whether or not the changes in lymphocyte subpopulations seen in aging humans are also found in aging dogs. Interrogating lymphocyte subpopulations requires fresh samples to be measured via flow cytometry, an assay much more technically involved than CBC, requiring specialized equipment and species-specific reagents. For this reason, we were only able to conduct flow cytometry analysis in the laboratory beagle cohort. Indeed, we observed that total T-cells, CD4 + T-cells, CD8 + T-cells and B cells declined with age in dogs, mirroring the trends reported in humans11,12. Furthermore, the ratio of CD4 + to CD8 + cells declined in geriatric dogs, an immune phenotype also observed in humans12. These results provide additional supporting evidence that changes in the immune system and immune cell populations are conserved elements of aging in dogs, mirroring age-related immune cell changes in humans10,11,12,17.
Insulin action, and subsequent downstream insulin signaling, is one of the most studied aspects of aging due to its integral hormonal functions regulating substrate utilization (e.g. glucose) and storage (e.g. fatty acids), both becoming aberrant with age in humans37,38. Studies in laboratory beagles and companion dogs have previously reported increases in fasting insulin and insulin resistance with age24,39. Our data show that geriatric laboratory beagles exhibit higher fasting insulin concentrations than middle aged beagles, recapitulating previous findings from other investigations. These elevated insulin concentrations with advanced age, albeit within the clinically normal range, may be indicative of deleterious metabolic changes impacting both healthspan and lifespan in dogs. Lifetime dietary restriction in labrador retrievers revealed that the preservation of insulin sensitivity and reduced insulin burden were associated with the delay of chronic disease and longer lifespan40. More research is needed to determine the impact of increased fasting insulin on deleterious health outcomes in dogs.
Adiponectin is another hormone that has been robustly investigated in the context of aging due to its association with age-related diseases and lifespan25. Our data demonstrate that adiponectin is significantly reduced in both aged and geriatric beagles, which has several physiological implications. One is the ability of adiponectin to promote insulin sensitivity and substrate utilization through AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor alpha (PPARɑ) in metabolic tissues. This reduction in circulating adiponectin may be contributing to the elevations in fasting insulin and reported insulin resistance observed in aged dogs24,41. Additionally, adiponectin has been reported to have profound anti-inflammatory effects through its ability to inhibit nuclear factor-kappa B (NF-κB) and thus reduce downstream tumor necrosis factor alpha (TNF-ɑ) and interleukin 6 (IL-6) production – all of which are generally associated with diseases of chronic inflammation42. Reduced adiponectin concentrations may contribute to the deterioration of immune function with age, however more research is needed to establish this relationship in dogs.
Our data demonstrate that dogs exhibit age-related changes in blood parameters and immune cell populations that are remarkably similar to those observed in humans. Specifically, we found that both lymphocyte and RBC counts decline with age in dogs, with corresponding decreases in hematocrit and hemoglobin levels in laboratory beagles. Notably, while human platelet counts tend to decline with age, we observed an increase in platelet counts in aging dogs. Moreover, our examination of lymphocyte subpopulations revealed that T-cells, B-cells, CD4 + T-cells and CD8 + T-cells all decline with age in dogs, accompanied by a lower CD4 to CD8 ratio in geriatric dogs, mirroring trends seen in elderly humans. Changes in these immune cell populations are also accompanied by alterations in insulin and adiponectin concentrations, which may be linked to deteriorated immune and metabolic function with age. These findings support that dogs, with their conserved age-related changes in blood and immune system parameters, represent a suitable model for studying human aging and age-related disease.
Study limitations
While CBC was measured in the large cohort of companion dogs, we were unable to explore any changes in certain lymphocyte populations via flow cytometry. Additionally, the number of beagles in the cohort was relatively small. Due to the observational nature of this study, there was no opportunity to further examine mechanisms or tissue specific immune populations. An attempt was made to quantify pro-inflammatory cytokine concentrations using a commercially available assay, however the assay failed internal quality control tests and proved unreliable. There were insufficient samples for repeating the analysis or utilizing another assay. Similarly, insufficient sample volume prevented lipid quantification in the Laboratory Beagle Study.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to data privacy or ethical restrictions.
References
-
Flurkey, K. & Currer, J. M. Pitfalls of animal model systems in ageing research. Best Pract. Res. Clin. Endocrinol. Metab. 18 (3), 407–421. https://doi.org/10.1016/j.beem.2004.02.001 (2004).
-
Creevy, K. E., Austad, S. N., Hoffman, J. M., O’Neill, D. G. & Promislow, D. E. The companion dog as a model for the longevity dividend. Cold Spring Harb Perspect. Med. 6 (1), a026633. https://doi.org/10.1101/cshperspect.a026633 (2016).
-
Kraus, C., Snyder-Mackler, N. & Promislow, D. E. L. How size and genetic diversity shape lifespan across breeds of purebred dogs. Geroscience 45 (2), 627–643. https://doi.org/10.1007/s11357-022-00653-w (2023).
-
McCoy, A. M. Animal models of osteoarthritis: comparisons and key considerations. Vet. Pathol. 52 (5), 803–818. https://doi.org/10.1177/0300985815588611 (2015).
-
Freeman, L. M. Cachexia and sarcopenia: emerging syndromes of importance in dogs and cats. J. Vet. Intern. Med. 26 (1), 3–17. https://doi.org/10.1111/j.1939-1676.2011.00838.x (2012).
-
Yadav, S. & Deepika, Maurya, P. M. A systematic review of red blood cells biomarkers in human aging. J. Gerontol. Biol. Sci. Med. Sci. ;79(4). (2024).
-
Pellegrino, R. et al. Temporal trends, sex differences, and age-related disease influence in neutrophil, lymphocyte count and neutrophil to lymphocyte-ratio: results from InCHIANTI follow-up study. Immun. Ageing. 20 (1), 46. https://doi.org/10.1186/s12979-023-00370-8 (2023).
-
Segal, J. B. & Moliterno, A. R. Platelet counts differ by sex, ethnicity, and age in the united States. Ann. Epidemiol. 16, 123–130. https://doi.org/10.1016/j.annepidem.2005.06.052 (2006).
-
Kubota, K. et al. [Changes in the blood cell counts with aging]. Nihon Ronen Igakkai Zasshi. ;28(4):509 – 14. Japanese. (1991). https://doi.org/10.3143/geriatrics.28.509
-
Schmid, S. M. et al. The companion dog as a model for inflammaging: a cross-sectional pilot study. Geroscience. Jun 1. (2024). https://doi.org/10.1007/s11357-024-01217-w. Epub ahead of print. PMID: 38822125.
-
Cepeda, S. & Griffith, A. V. Thymic stromal cells: roles in atrophy and age-associated dysfunction of the thymus. Exp. Gerontol. 105, 113–117. https://doi.org/10.1016/j.exger.2017.12.022 (2018).
-
Olsson, J. et al. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121 (1–3), 187–201. https://doi.org/10.1016/s0047-6374(00)00210-4 (2000).
-
Yu, Y. et al. B Cells Dynamic in Aging and the Implications of Nutritional Regulation. Nutrients. ;16(4):487. (2024). https://doi.org/10.3390/nu16040487. PMID: 38398810.
-
Sansoni, P. et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood 82 (9), 2767–2773 (1993).
-
Vergori, A. et al. Orchestra study. SARS-CoV-2 mRNA vaccine response in people living with HIV according to CD4 count and CD4/CD8 ratio. Vaccines (Basel). 11 (11), 1664. https://doi.org/10.3390/vaccines11111664 (2023). On Behalf Of The Vax-Icona-.
-
Castilho, J. L. et al. North American AIDS cohort collaboration on research and design (NA-ACCORD) of the international epidemiology databases to evaluate AIDS (IeDEA). CD4/CD8 ratio and Cancer risk among adults with HIV. J. Natl. Cancer Inst. 114 (6), 854–862. https://doi.org/10.1093/jnci/djac053 (2022).
-
Greeley, E. H. et al. The influence of age and gender on the immune system: a longitudinal study in Labrador retriever dogs. Vet. Immunol. Immunopathol. 82 (1–2), 57–71 (2001).
-
Greeley, E. H., Kealy, R. D., Ballam, J. M., Lawler, D. F. & Segre, M. The influence of age on the canine immune system. Vet. Immunol. Immunopathol. 55 (1–3), 1–10 (1996).
-
McKenzie, B. et al. Changes in insulin, adiponectin and lipid concentrations with age are associated with frailty and reduced quality of life in dogs. Sci. Rep. 15, 5380. https://doi.org/10.1038/s41598-025-89923-z (2025).
-
Li, N. et al. Adiponectin preserves metabolic fitness during aging. Elife 10, e65108. https://doi.org/10.7554/eLife.65108 (2021).
-
Preuss, H. G. Effects of glucose/insulin perturbations on aging and chronic disorders of aging: the evidence. J. Am. Coll. Nutr. 16 (5), 397–403. https://doi.org/10.1080/07315724.1997.10718704 (1997).
-
Chen, Y. H. et al. Association between high-fasting insulin levels and metabolic syndrome in non-diabetic middle-aged and elderly populations: a community-based study in Taiwan. BMJ Open. 8 (5), e016554. https://doi.org/10.1136/bmjopen-2017-016554 (2018).
-
Marmentini, C. et al. Aging reduces insulin clearance in mice. Front. Endocrinol. (Lausanne). 12, 679492. https://doi.org/10.3389/fendo.2021.679492 (2021).
-
Bhashyam, S. et al. Aging is associated with myocardial insulin resistance and mitochondrial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 293 (5), H3063–H3071. https://doi.org/10.1152/ajpheart.00163.2007 (2007).
-
Iwabu, M., Okada-Iwabu, M., Yamauchi, T. & Kadowaki, T. Adiponectin/adiponectin receptor in disease and aging. NPJ Aging Mech. Dis. 1, 15013. https://doi.org/10.1038/npjamd.2015.13 (2015).
-
Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N, Pedersen AA, Kalthoff C,Tullin S, Sams A, Summer R, Walsh K. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem. 2010;;285(9):6153-60. doi: 10.1074/jbc.M109.088708.
-
Arai, Y., Kamide, K. & Hirose, N. Adipokines and aging: findings from centenarians and the very old. Front. Endocrinol. (Lausanne). 10, 142. https://doi.org/10.3389/fendo.2019.00142 (2019).
-
Torrente, C. et al. Adiponectin as a sepsis biomarker in dogs: diagnostic and prognostic value. Vet. Clin. Pathol. 49 (2), 333–344. https://doi.org/10.1111/vcp.12858 (2020).
-
Kim, H. S., Kang, J. H., Jeung, E. B. & Yang, M. P. Serum concentrations of leptin and adiponectin in dogs with myxomatous mitral valve disease. J. Vet. Intern. Med. 30 (5), 1589–1600. https://doi.org/10.1111/jvim.14570 (2016).
-
Chen, F. L. et al. Evaluating instruments for assessing healthspan: a multi-center cross-sectional study on health-related quality of life (HRQL) and frailty in the companion dog. Geroscience 45 (4), 2089–2108. https://doi.org/10.1007/s11357-023-00744-2 (2023).
-
Sánchez-Solé, R., Pedreira, G., Venzal, J. M., Fonseca-Alves, C. E. & Serdio, P. P. The use of flow cytometry for diagnosis and immunophenotyping in chronic lymphocytic leukemia in a dog: clinical case report. Open. Veterinary J. 12 (6), 868–876. https://doi.org/10.5455/OVJ.2022.v12.i6.13 (2022).
-
Vanhooren, V. & Libert, C. The mouse as a model organism in aging research: usefulness, pitfalls and possibilities. Ageing Res. Rev. 12 (1), 8–21. https://doi.org/10.1016/j.arr.2012.03.010 (2013).
-
Jones, C. I. Platelet function and ageing. Mammalian Genome: Official J. Int. Mammalian Genome Soc. 27 (7–8), 358–366. https://doi.org/10.1007/s00335-016-9629-8 (2016).
-
Gholizade, M. et al. Association between platelet, white blood cell count, platelet to white blood cell ratio and sarcopenia in community-dwelling older adults: focus on Bushehr elderly health (BEH) program. BMC Geriatr. 22 (1), 300. https://doi.org/10.1186/s12877-022-02954-3 (2022).
-
Lee, H. S., Koh, I. H., Kim, H. S. & Kwon, Y. J. Platelet and white blood cell count are independently associated with sarcopenia: A nationwide population-based study. Thromb. Res. 183, 36–44. https://doi.org/10.1016/j.thromres.2019.09.007 (2019).
-
Ligthart, G. J., Schuit, H. R. & Hijmans, W. Subpopulations of mononuclear cells in ageing: expansion of the null cell compartment and decrease in the number of T and B cells in human blood. Immunology 55 (1), 15–21 (1985).
-
Chang, A. M. & Halter, J. B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 284 (1), E7–12. https://doi.org/10.1152/ajpendo.00366.2002 (2003).
-
Carpentier, A. C. 100th anniversary of the discovery of insulin perspective: insulin and adipose tissue fatty acid metabolism. Am. J. Physiol. Endocrinol. Metab. 320 (4), E653–E670. https://doi.org/10.1152/ajpendo.00620.2020 (2021).
-
Mori, N., Kawasumi, K. & Arai, T. Comparison of the plasma insulin and adiponectin concentrations as metabolic markers in clinically healthy dogs with ageing. J. Anim. Vet. Adv. 11, 971–974 (2012).
-
Larson, B. T., Lawler, D. F., Spitznagel, E. L. Jr & Kealy, R. D. Improved glucose tolerance with lifetime diet restriction favorably affects disease and survival in dogs. J. Nutr. 133 (9), 2887–2892. https://doi.org/10.1093/jn/133.9.2887 (2003).
-
Ruan, H. & Dong, L. Q. Adiponectin signaling and function in insulin target tissues. J. Mol. Cell. Biol. 8 (2), 101–109. https://doi.org/10.1093/jmcb/mjw014 (2016).
-
Ajuwon, K. M. & Spurlock, M. E. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288 (5), R1220–R1225. https://doi.org/10.1152/ajpregu.00397.2004 (2005).
Author information
Authors and Affiliations
Contributions
FLC designed and managed the companion dog study. JEM, MN and EEM designed and managed the laboratory beagle study. JEM, MN and EEM were involved in study operations and data acquisition. JEM, JLG, APT and MP drafted the manuscript and carried out the analysis of the data. JLG performed statistical analyses. EEM, MP, KG, CLHH and DJS provided intellectual support. JEM, JLG, APT, MP, KG and DJS were involved in writing the manuscript. All authors had final approval.
Corresponding author
Ethics declarations
Competing interests
All authors are current or former employees of Cellular Longevity, Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
McMahon, J.E., Graves, J.L., Tovar, A.P. et al. Translational immune and metabolic markers of aging in dogs.
Sci Rep 15, 14460 (2025). https://doi.org/10.1038/s41598-025-99349-2
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-99349-2
This post was originally published on this site be sure to check out more of their content.