Abstract
Chronic enteropathy (CE) is a common complaint in canine gastroenterology. Recently, faecal microbiota transplantation (FMT) gained attention as a treatment strategy. However, the efficacy and long-term impact of FMT is still unclear. Clinical index (CIBDAI), faecal microbiota and metabolome were monitored in 20 CE dogs refractory to diet before (T0) and 3 months (T3) after FMT. Further data were retrospectively collected up to 1-year after FMT. Significant improvements were observed in CIBDAI, Dysbiosis Index (DI), and primary (PBAs) and secondary (SBAs) faecal bile acids and propionate one month (T1) after FMT (CIBDAI (median and range): T0 5 (1–9) vs. T1 1 (0–5), p < 0.0001; DI (median and range): T0 -0.1 (-5.6 to 3.8) vs. T1 -2.1 (-5.7 to 4.7), p < 0.05; PBAs decreased by 57%, SBAa increased by 41%; propionate increased by 20%). According to CIBDAI, 17 dogs clinically improved up to T3, and 10 dogs remained clinically stable up to one year after FMT. Alpha- and beta-diversity of the faecal microbiota of CE dogs did not differ, neither before nor after FMT, from that of 17 healthy controls. The results highlight that CE dogs refractory to diet with mild clinical signs and dysbiosis may benefit long-term from treatment with FMT.
Introduction
Chronic enteropathy (CE) is a common presenting complaint in dogs in veterinary practice. CE is characterised by chronic persistent or recurrent gastrointestinal signs, such as diarrhoea, vomiting, hyporexia, abdominal discomfort and potentially weight loss lasting longer than 3 weeks1. CE includes different clinical phenotypes, which are traditionally classified according to the clinical response to sequential treatments performed stepwise to achieve clinical remission2. Given the high response rate to empirical dietary management, diet change is considered the first line therapeutic approach to CE dogs, and a variety of diet types are available for this purpose3. Adjunctive or subsequent therapeutic interventions can be considered in dogs with CE, including antimicrobials, and immunosuppressive drugs1. Recently, the appropriateness of routine empirical antimicrobial treatment trials has been questioned, therefore alternative strategies targeting microbiota modulation, including faecal microbiota transplantation (FMT), have been proposed4,5. FMT involves transferring intestinal contents from a healthy donor to a diseased recipient to treat conditions mainly related to the gastrointestinal tract6,7. FMT has been largely and successfully used in treating antibiotic-refractory Clostridioides difficile infections in humans8. Recently, FMT has gathering attention in veterinary practice as well. A recent survey of practitioners identified indications for FMT use in treating CE, parvovirus infection and acute diarrhoea, with mixed to good clinical response and rare side effects9. New guidelines for FMT in companion animals have been proposed recently6. The safety of FMT has been investigated in healthy dogs and reported side effects were mild and transitory gastrointestinal signs10. The same has been sporadically described in diseased dogs receiving FMT11, or no side effects have been reported12,13,14. In addition to clinicians-reported experience and case reports9,12,13,14,15, the clinical efficacy of FMT as adjunctive therapy in dogs with refractory CE was reported recently in a retrospective study11. Prospective studies published so far either included a few dogs or did not standardise the route for FMT administration16,17.
Based on retrospective data collection or case reports, we hypothesized that FMT may be an effective treatment to achieve clinical improvement in CE dogs, when used as a primary treatment.
This study aimed to prospectively evaluate the response up to 3 months on clinical outcome and the composition of the faecal microbiota of CE dogs refractory to diet based on lack of response to at least two dietary trials, one of which was conducted at the time of enrolment. Dogs were treated with FMT as a primary treatment by retention enema. Once the prospective study was concluded, additional data on a long-term outcome were retrospectively collected up to 1-year post-FMT.
Materials and methods
Study participants
All the dogs involved in the study were referred to the Units of Gastroenterology at the Veterinary Teaching Hospital of the University of Bologna (UNIBO, Italy) and the Internal Medicine Unit of the private referral hospital Anicura Ospedale Veterinario I Portoni Rossi (OVIPR, Italy).
In this prospective, longitudinal, multi-centric study, privately owned dogs were considered if presented with chronic (≥ three weeks duration) idiopathic GI signs (diarrhoea, vomiting and/or nausea, abdominal bloating and/or cramps, or a combination thereof), with no or partial response to at least two elimination diet trials, one of which lasted two weeks. Extra-intestinal disease was ruled out by diagnostic work-up including haematology, serum chemistry, and faecal flotation with antigen-testing for giardiasis, and abdominal ultrasonography. Exclusion criteria were the administration of antibiotics or other medical treatments, except for cobalamin, within 30 days before FMT. However, a recent antibiotic history (up to 30 days before the enrolment) was taken into account for comparisons with non-recent treatment (any antibiotics treatment stopped up to 60 days before enrolment or no treatment declared). Dogs that were undergoing cobalamin therapy continued to receive it throughout the study.
The dogs included in the study were patients referred to the two centres. All dogs had undergone at least one dietary trial previously at the referring institution. After referral, as a part of the study protocol, a further 2-weeks diet trial was conducted. The choice of the diet was made according to the patient’s nutritional assessment and the owner’s preferences, leading to either 1) one of 3 commercial therapeutic diets based on soybean or fish hydrolysed protein source (Purina Pro Plan Veterinary Diets Hypoallergenic HA, Royal Canin Veterinary Diet Hypoallergenic, or Farmina Vet Life UltraHypo) all of which were different from the ones previously tried, or 2) a home-made diet based on a novel animal protein and carbohydrate source (horse meat and potatoes).
Dogs that failed to respond to the diet change after two weeks, based on the lack of full recovery from the presenting GI signs, were then classified as non diet-responsive and eligible to be enrolled in this study. Dogs were continued on the same diet as during the 2-weeks diet trial for the remaining duration of the study.
Healthy dogs
Faecal samples were collected from 17 clinically healthy dogs and used for microbiota analysis for comparison to the dogs with CE. All dogs were deemed clinically healthy based on lack of gastrointestinal signs or other diseases for the past 6 months based on owner information. The relevant data about their age, including both normal DI and P. hiranonis faecal abundance are available in Supplementary Table S1.
FMT donors
Two healthy privately owned spayed female adult dogs, aged 4 and 6 years old, with normal body weight, served as donors (one per facility). According to published guidelines6,7, donors had not been on antibiotics or other medical treatments in the previous 12 months, had a complete diagnostic work-up and were screened for enteropathogens (C. difficile, CPV2, C. perfringens net F toxin gene, Campylobacter jejuni, Salmonella spp.). The donors had a faecal Dysbiosis Index (DI)18 of -5.5 and -5.6, respectively.
FMT protocol
Fresh faeces sampled from donors on the same day of each FMT procedure were suspended in 0.9% sodium chloride solution in a 1:2 w/v ratio and mixed using a laboratory blender within 1 h from sampling. The faecal solution was filtered through a medical gauze pad and distributed into 60 ml sterile syringes to be administered by enema using PVC catheters (16–21 F according to the dog’s size). As recommended by published guidelines6,7, FMT was administered at the dosage of 2.5–5 g faeces per kg/body weight (BW) of the recipient by the end of the same day of donor sampling. After FMT, dogs were kept on cage rest for two hours to prevent early defecation.
Study design
One to two FMT(s), at the discretion of the attending clinicians, were administered to dogs through retention enema within two weeks (T0). Then, a short-term follow-up evaluation was performed at, 30 (T1), 60 (T2) and 90 (T3) days after the last FMT. The decision to administer—or not – a further FMT was made based on the clinical improvement shown after the first procedure. Dogs were required to continue the diet used for the diet trial. At T0, T1, T2 and T3 dogs were clinically re-evaluated and body weight (BW), Body Condition Score (BCS 1–9 scale19), and the Canine IBD Activity Index (CIBDAI20 ) were recorded.
A long-term follow-up was retrospectively collected by obtaining data from the last clinical check-up performed at the facility where the dog had undergone FMT (UNIBO, OVIPR). Dogs that had a clinical follow-up at least 6 months after faecal transplantation (T0) were included. Based on data reported on medical records, the dogs were categorized according to the duration of the time-to-relapse from the FMT, intended as the worsened CIBDAI for at least one point from the value recorded at the last short-term follow-up (T3).
Faecal samples
Faecal samples were collected at T0, T1, T2 and T3 for chemical and microbial analysis, and the evaluation of the Faecal Score (Faecal Scoring System, Nestle Purina Petcare). The owners collected faecal samples on the same day of the clinical evaluation. The samples were stored at − 80 ºC at UNIBO site immediately after the delivery, while the samples from OVIPR were stored locally at − 20 ºC and shipped within a week to UNIBO to be stored long-term at − 80 ºC.
Faecal samples were divided into two aliquots, and one was shipped in dry ice to the Gastrointestinal Laboratory of Texas A&M University, College Station, Texas, United States, for fecal bile acids, faecal targeted metabolomics, qPCR-based Dysbiosis Index, DNA shotgun sequencing, and concentrations of fecal calprotectin. Faecal pH, ammonia and volatile fatty acids (VFA) were measured at the Laboratory of Animal production of University of Bologna, Ozzano dell’Emilia, Italy.
Faecal calprotectin
Calprotectin was measured in all faecal extracts using the fCal turbo assay (Buhlmann kit), on an automated analyser (AU 480; 220 Olympus/Beckman Coulter, Brea, CA, United States), based on a published method21.
Faecal metabolomics analysis
The faecal pH was measured following a 1:10 (w/v) dilution with deionized water using a laboratory pH meter (SevenMulti, Mettler Toledo, Greinfesee, Switzerland; accuracy ± 0.01). Faecal ammonia was assessed utilizing an enzymatic colorimetric test (Urea/BUN-Color; BioSystems S.A., Barcelona, Spain) and expressed as µmol/g of faeces. VFA were quantified via gas chromatography, according to a method described previously22 and were expressed as % of total measured VFA. Faecal concentrations of unconjugated bile acids (BA) and sterols were measured by a previously described gas chromatography with mass spectrometry assay performed as previously reported23,24.
Faecal microbiome analysis
DNA was isolated from a 100 mg sample of faeces using the MagMAX™ CORE kit and MagMAX™ CORE Mechanical Lysis Module with Kingfisher Flex system (Kingfisher Biotech, MN, USA) following the manufacturer’s instructions.
Faecal DNA aliquots were submitted to Diversigen (New Brighton, MN, USA) for shallow DNA shotgun metagenomic sequencing as previously described25. Shortly, DNA libraries were prepared using a protocol adapted from the Nextera XT kit (Illumina, San Diego, CA, USA) and sequenced on an Illumina NovaSeq 6000 platform, generating paired-end 2 × 150 bp reads with a target depth of approximately 2 million reads per sample in that sequencing batch. Quality control included positive and negative controls on each library preparation plate. Raw sequences were filtered to remove low-quality reads (Q-score < 30) and read shorter than 50 bp, with adapter sequences trimmed using Cutadapt. Host sequences were excluded using Bowtie2, and sequences were trimmed to a maximum length of 100 bp and converted to a single FASTA format using shi7. DNA sequences were aligned to a curated database (DivDB-Canine) containing all representative bacterial genomes from RefSeq, supplemented with manually curated strains, using BURST with 97% identity. Taxonomic assignments were determined by the lowest common ancestor method, consistent across at least 80% of reference sequences for each input sequence. Operational Taxonomic Units (OTUs) with low representation (< 0.01% of unique genome regions and < 1% of the whole genome) were excluded, and OTU counts were normalised to average genome length and converted to relative abundance for each sample. Downstream analysis was conducted using QIIME 2 (version 2021.11), with samples rarefied to 156,162 reads.
The DI was conducted according to the methodology outlined in the study by AlShawaqfeh et al.18 The qPCR panel comprised eight bacterial groups: total bacteria, Faecalibacterium spp., Turicibacter spp., Escherichia coli, Streptococcus spp., Blautia spp., Fusobacterium spp., and Peptoacetobacter (Clostridium) hiranonis. The qPCR results were presented as the logarithmic quantity of DNA (fg) for each specific bacterial group per 10 ng of total isolated DNA. The abundance of these bacterial groups was utilized to compute the DI using a validated mathematical algorithm18. DI results were further categorized into four subgroups: normal (DI < 0, with all targeted bacteria within reference interval (RI)), minor shift (DI < 0, with one or more targeted bacteria outside the RI), mild to moderate shift (0 < DI < 2), and significant dysbiosis (DI ≥ 2)25.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The study was approved by the Ethics Committee of the University of Bologna (ID 1210 protocol number 105657 30/04/2021). The study was carried out with adherence to a high standard (best practice) of veterinary care. Before being enrolled into the study owners of each dog signed a written informed consent form. All methods were performed in accordance with the relevant guidelines and regulations. According to the editorial policy for Scientific Reports, the study was carried out in compliance with the ARRIVE guidelines.
Statistical analysis
The normality of patient demographics, clinical features, faecal chemical and qPCR-related data were assessed using the D’Agostino-Pearson test.
Data were analysed as the interaction between the time before FMT (T0) and each follow-up at 30 (T30), 60 (T60), and 90 (T90) days after FMT by a mixed effect analysis with Tukey post-test. Data for the Dysbiosis Index and Peptoacetobacter hiranonis abundance were grouped in AB- and NOT-AB group, according to a previous antibiotic administration, and analysed by unpaired t-test or Mann–Whitney test. Spearman’s analysis was performed to test correlation between P. hiranonis abundance and primary or secondary BA.
Alpha diversity metrics, including Shannon, Chao1, and observed features, were calculated, while beta diversity was assessed using Bray–Curtis distances and visualized with principal coordinate analysis (PCoA) plots. Analysis of similarities (ANOSIM) and permutational multivariate analysis of variance (PERMANOVA) were used for statistical analysis of beta diversity. Taxonomy was evaluated using analysis of compositions of microbiomes (ANCOM)26 and Analysis of compositions of microbiomes with bias correction (ANCOM-BC)27 for differential abundance analysis.
Data are reported as median (range) or mean ± SD. For microbiome analyses, significance was set at P < 0.05 after adjustment for multiple comparisons using the Benjamini–Hochberg calculation. Statistical analyses were performed using GraphPad Prism version 9.2 (GraphPad Software, San Diego, CA, USA) and R studio (package: ANCOMBC and phyloseq)27,28 .
Results
During the study period, twenty dogs met the inclusion criteria. Patient demographics (median, range) were as follows: age (months 41, 10–118); body weight (kg 24, 3.5–50); BCS19 (4, 3–6). Most of them were purebred dogs (17/20), with 16 different breeds represented: two were Boxer dogs, and the remaining dogs were American Staffordshire Terrier, Staffordshire Bull Terrier, German Shepherd, Beagle, Golden Retriever, Newfoundland, Maltese dog, English setter, Dobermann, Weimaraner, Yorkshire Terrier, Chihuahua, Rhodesian Ridgeback, Basset Hound, and Poodle. Among them, 15/20 were male, of which 11 were intact and 4 were castrated, and 4/20 were female, of which three were intact and one was spayed.
Clinical evaluations
At T0, diarrhoea was the most commonly reported clinical sign for 18/20 dogs, followed by vomiting and/or manifestation of nausea (8/20), abdominal bloating and/or abdominal pain (6/20). Six dogs had a history of pruritus or dermatologic diseases. These relevant symptoms are not taken into account by the CIBDAI scoring system.
Out of the 20 dogs included, 13 did not report antibiotic use in the 60 days before study inclusion (NOT-AB group). Seven dogs had undergone antibiotic therapy (6/7 metronidazole, 1/7 enrofloxacin) between 30 to 60 days before T0 (AB group). Dogs in the NOT-AB group had a mean age of 37 ± 38 months, which did not differ from the AB group, having a mean age of 52 ± 35 months (P = 0.38). At timepoint 0, the CIBDAI was not statistical different between NOT-AB group (median 4; range 1–9) vs AB group (median 4; range 3–7 P = 0.6).
Diet and cobalamin status
Dogs were fed either one of three different commercial hydrolysed protein diets (12/20) or a home-made diet based on a novel protein source (8/20). The diet trial before FMT corresponded respectively to the 2nd attempt for 13 dogs and the 3rd attempt for 7 dogs. At total of 9/20 dogs received oral cobalamin supplementation. Only two dogs had cobalamin level below reference range (234 ng/L) with both undetectable values (the detection limit of 150 ng/L) and both were receiving supplementation. Another 7 dogs had cobalamin level < 400 ng/L documented at T0, and therefore cobalamin was supplemented based on current recommendations. At T0, average serum cobalamin level was 374 (150–780) ng/L; in dogs receiving supplementation, cobalamin was 264 (range 150–374) ng/L versus 532 (360–780) ng/L in dogs not receiving it.
FMT and clinical response
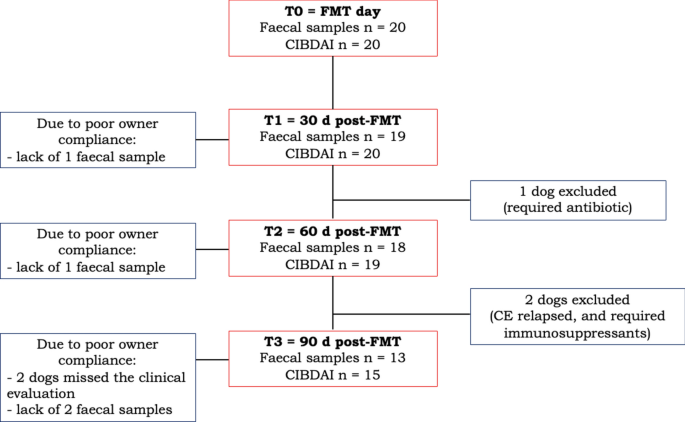
At T0, 8/20 dogs received one FMT, while 12/20 dogs received two FMTs. For all dogs, sedation was not required, and no adverse events were observed or reported during or after the procedure. After FMT, clinical evaluations were available for 20 dogs at T1, 19 dogs at T2, and 15 dogs at T3; faecal samples were available for 19 dogs at T1, 18 dogs at T2, and 13 dogs at T3 (Fig. 1). Between T1 and T2, one dog was excluded from the study due to a disease not related to CE, that required antibiotic treatment. However, besides the development of pericarditis, the dog did show clinical improvement after FMT. At T3 due to the owners’ lack of compliance, the stool sample from one dog was missed, while two dogs skipped the appointment at the clinic, and their owners justified that the dogs were fine as per T2. Based on this, no CIBDAI, BW or BCS were recorded for these two dogs. An increased BW (median and range) was observed after FMT (T0 = 24 (3.5–50) vs. T1 = 26 (3.4–52, P = 0.022, Supplementary Figure S1), with no impact on the BCS (T0 and T1 = 4 (3–6), P > 0.05).
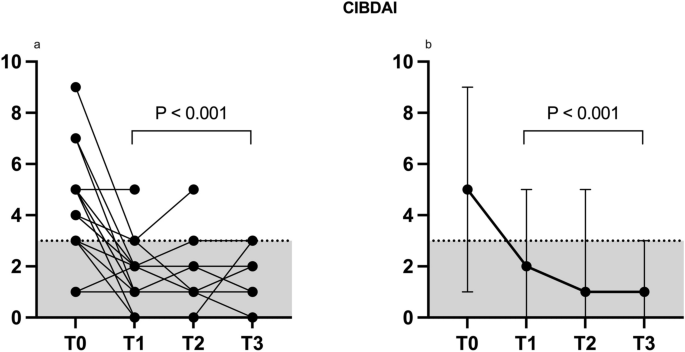
At T0, CIBDAI (Fig. 2a, median and range 2b,) was 5 (1–9) and then decreased to 1 up to T3 (T1 and T2 range: 0–5; T3: 0–3; T1, T2 and T3 were all different from T0 with a p < 0.0001). In summary, 17/20 dogs improved up to 3 months after FMT (T3). Among the dogs (3/20) that did not show clinical improvement, two dogs required endoscopic examination and exclusion from the study for worsening of gastrointestinal symptoms. However, one of these two dogs showed an initial improvement in CIBDAI at T1 (T0 = 7 vs. T1 = 1), while the other did not (T0 = 4 vs. T1 = 4). Another dog did not respond to FMT according to CIBDAI, but the clinical signs continued to be mild after FMT, mainly related to poor faecal consistency, therefore the exclusion from the study was not necessary (CIBDAI at T0 = 2 vs. T1 = 2). This dog was one of the two having a cobalamin level below the reference range.
CIBDAI index assessed in 20 CE dogs (all values a, median and range b) before (T0) and 30 (T1), 60 (T2) and 90 (T3) days after FMT. Values up to 3 are indicative of clinically insignificant disease20.
With regard to the long-term follow-up collected retrospectively among dogs which responded to FMT, 7/17 dogs presented at least one relapse, intended as an increase of the CIBDAI index from baseline, for at least one time point, in a period between 6 and 12 months after FMT, while no relapse was recorded for the remaining 10 dogs up to 12 months after FMT. The CIBDAI recorded at T0 was not different between those who relapsed (4, 3–7) and those who did not (4,3–9).
Microbiome results
qPCR
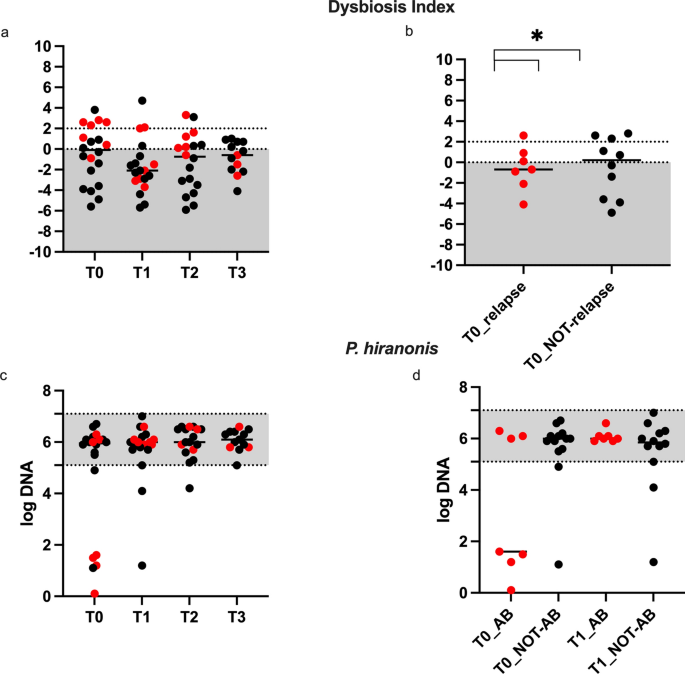
The DI values are shown in Figs. 3a-b. At T0, dogs had a median (range) DI of -0.1 (-5.6 to 3.8), and 50% of dogs had a DI > 0, of which 6/10 dogs were in the AB group. At T0, no difference in age (months, mean ± SD) was seen between dogs with a DI < 0 (43.7 ± 21.7) and DI > 0 (50.3 ± 46.8). No differences were observed in DI before and after FMT (P > 0.05). From T0 to T1 median DI decreased to -2.1 (range -5.7 to 4.7), to -0.7(-5.9 to 3.3) at T2, and to -0.6 (-4.1 to 1) at T3. At T0, dogs in the AB group had a DI of 2.3 (-0.9 to 2.8) compared to dogs in the NOT-AB group with a DI of –1.4 (-5.6 to 3.8) (P = 0.01) (Fig. 3B). For those dogs with a history of antibiotic treatment, a significant difference in DI was observed between T0 2.3 (-0.9 to 2.8) and T1 -2.1 (-3.7 to 2.1) (P = 0.023). No significant differences were observed between T0 vs T2 and T3 in the AB group and no differences were observed in the NOT-AB group at any time compared to T0 (P > 0.05).
Dysbiosis Index (a-b) and Peptoacetobacter hiranonis abundance (log DNA, c-d) assessed in 20 CE dogs before (T0) and 30 (T1), 60 (T2) and 90 (T3) days after FMT. The grey shaded area is the reference abundance for P. hiranonis, and values < 0 indicate normal DI, > 0 and < 2 indicate mild changes in bacterial populations18. The red dots represent dogs in the (ab) group receiving antibiotic therapy 30 to 60 days before FMT (T0). *P < 0.05.
Of the 20 included dogs, 10 had a DI > 0 at the time of inclusion, and 10 had a DI < 0. At T1, the DI decreased below 0 in 5/10 dogs having an initial DI > 0, and 4/5 were in the AB group. A significant age difference (in months, mean ± SD) was observed between dogs with a decrease in DI < 0 (16 ± 7) and those with a DI > 0 (84 ± 45) after FMT at T1 (P = 0.002). Among dogs in the AB group with an initial DI > 0, in 4/6 dogs the DI decreased below 0 at T1, but the DI at T1 was not different between dogs (P = 0.05). At T0, the DI of dogs that clinically relapsed within 6–12 months from FMT (-0,7, -4,1 to 2,6) was not different (P > 0.05) from that of dogs that did not relapse (0,2, -4,9 to 2,8).
Data on Peptoacetobacter hiranonis are shown in Fig. 3c-d. No overall differences in P. hiranonis abundance were observed before and after FMT (P > 0.05). However, at T0, 6 dogs had P. hiranonis abundance below the reference range (Fig. 3c), and among these, the 4 dogs in the AB group fully recovered after FMT (Fig. 3d), even though the difference was not significant (P = 0.05).
DNA shotgun sequencing
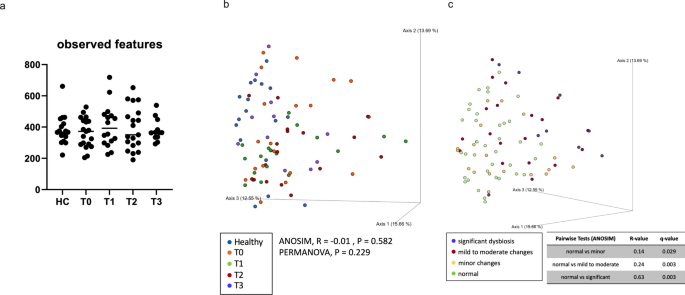
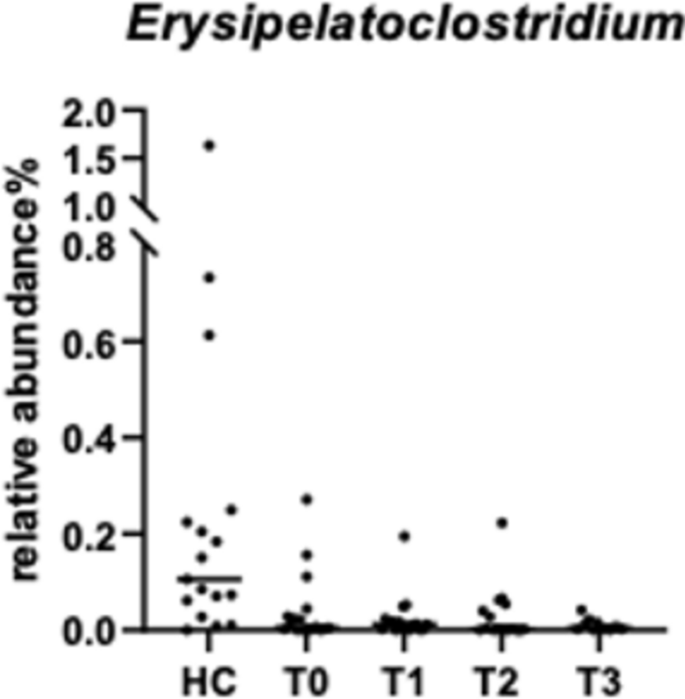
Due to poor DNA quality, samples from one dog at T0, T1, and T3, and one sample from another dog at T2 were excluded from the sequencing analysis. For alpha-diversity (Fig. 4a), no significant differences were observed between the four time points in dogs with CE refractory to diet and healthy controls (n = 17). Beta diversity based on Bray–Curtis distance (Fig. 4b) also did not differ between groups, according to both ANOSIM (P = 0.6) and PERMANOVA (p = 0.2) analyses. However, when samples were categorized based on normal DI, minor changes, mild to moderate changes, and significant dysbiosis25, differences between dogs with CE refractory to diet and healthy controls were found (ANOSIM, R = 0.25, P = 0.001, n = 83, Fig. 4c). PERMANOVA test also confirmed the differences between four different categories (P = 0.001). According to ANCOM, the genus Erysipelatoclostridium was the only differentially abundant bacterium, with a W value of 187. Specifically, dogs with CE had a lower abundance of Erysipelatoclostridium spp. compared to healthy control dogs (Fig. 5). No bacterial groups were differentially abundant between groups based on ANCOM-BC analysis.
(a) Alpha diversity (observed feature) and (b) Beta diversity (Bray–Curtis distance) in healthy dogs (HC) and dogs with CE assessed before (T0) and 30 (T1), 60 (T2) and 90 (T3) days after FMT; (c) Beta diversity according to Bray–Curtis distance categorized based on the interpretation of the Dysbiosis Index (DI)25 in 20 dogs with CE and 17 healthy dogs.
Faecal metabolome
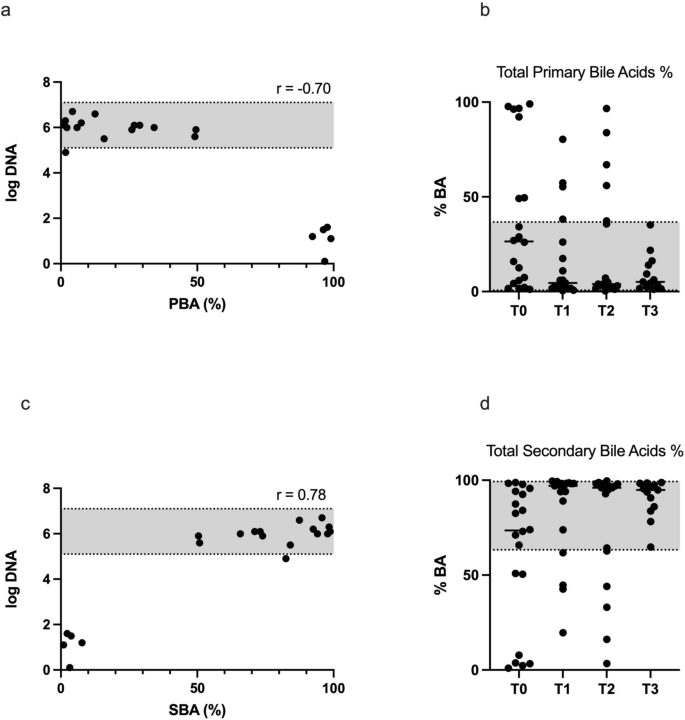
Faecal bile acids (primary: PBAs, and secondary: SBAs, Table 1 and Fig. 6b and 6d) concentrations (% of total bile acids) significantly differed in response to FMT at T1 (P < 0.05), while no differences were observed between T0 and T2 or T3 (Table 1). After FMT at T1, total PBAs decreased by 57%, and among PBAs, cholic acid decreased by 58%, and chenodeoxycholic acid by 55%. Contrary to PBAs, total SBAs increased by 41%, and among SBAs, lithocholic acid increased by 58% and deoxycholic acid by 34%. At T0, P. hiranonis significantly correlated with total (%) PBAs (r = -0.698, P < 0.001) and SBAs (r = 0.775, P < 0.0001), as shown in Fig. 6a and 6c. No correlation was observed at any other time point, likely because too few samples were abnormal at these time points.
(a and c): Spearman correlation between the abundance of P. hiranonis (Log DNA) in 20 CE dogs before FMT (T0) and total (%) primary bile acids (PBA, 6A) and secondary bile acids (SBA, 6C). The grey shaded area is the reference abundance for P. hiranonis, (b) and (d): Faecal primary and secondary bile acids concentrations (6b and d, %) assessed before (T0) and 30 (T1), 60 (T2) and 90 (T3) days after FMT. The grey shaded area is the reference abundance. In both graphs, T0 vs. T1 was significantly different (P < 0.01).
Faecal pH, ammonia and sterols did not differ in response to FMT (P > 0.05, Supplementary Table S2). Among faecal volatile fatty acids (VFA, Supplementary Table S3), the concentration of propionic acid (median and range, % of total VFA) increased by 20% at T1 compared to T0 (26.0, 5.5–47 vs. 32, 3.5–47, P = 0.0929), while acetic, isobutyric, n-butyric and isovaleric acid did not change in response to FMT (Supplementary Table S3).
Faecal calprotectin
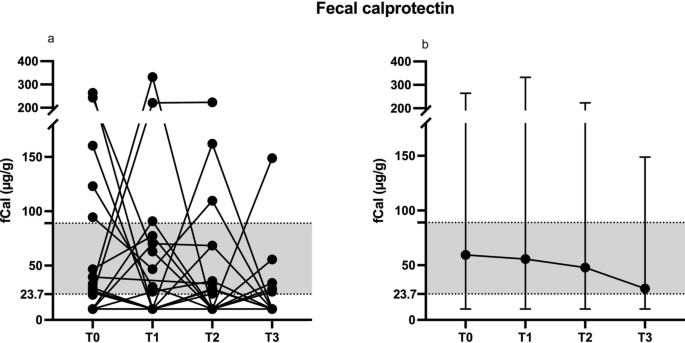
The faecal calprotectin (fCal) concentrations (μg/g, median and range, Fig. 7 a and b) showed no significant differences (P > 0.05) between T0 (26, 0–264), T1 (22, 0–332), T2 (28, 0–223), and T3 (5.9, 0–149). All dogs with abnormal fCal levels at T0 (n = 5) showed fCal within the range after FMT at T1, but 3/5 dogs reverted to the previous state between T2 and T3. Two dogs presented with fCal values above the range after FMT at T1, and one did not clinically improve after FMT, requiring additional immunosuppressive therapy before T3.
The faecal calprotectin levels (μg/g all values a, median and range b) assessed before (T0) and 30 (T1), 60 (T2) and 90 (T3) days after FMT. The grey shaded area represents the reference interval for the assay. The Minimum Quantification Level (MQL) is 23.7 μg/g, and values < MQL are set to 10 in the graph.
Discussion
In this prospective, longitudinal, multi-centric study, the clinical outcome, as well as the effects observed on faecal microbiota and metabolome of 20 dogs with CE either non-responsive or only partially responsive to at least two dietary trials and treated with FMT as a primary treatment following the diet change, are described.
Dogs included in this study exhibited characteristics previously documented in those with chronic gastrointestinal signs, including a higher prevalence in males over females, a median age of around 4 years and a body weight of approximately 20 kg29,30,31. The dietary treatment was unchanged throughout the study and consisted of a hydrolysed protein diet in 60% of dogs, while the remaining dogs were fed a home-cooked diet. Dietary intervention used as a monotherapy has been proven to induce remission in the majority of CE dogs29,30,32,33,34, and there is evidence that if there is no response to changing the diet, a second attempt with a different diet is recommended35. Therefore only dogs which failed to sufficiently respond at least to two different dietary treatments were considered candidates to receive FMT as the subsequent treatment approach5.
Dogs were clinically scored throughout the study using the CIBDAI score20, a standardised method which, as it only considers six clinical signs, may prove to be limited in certain cases, as in this study where relevant symptoms such as nausea, abdominal distension and pain were not included.
Before being enrolled in the study, 9/20 dogs were receiving oral cobalamin supplementation, although only two dogs were below reference interval. The reason for this was the current recommendation that cobalamin levels less than 400 ng/L should be corrected36, therefore seven dogs received the supplementation despite values in the low range of normality, as it would not have been ethically feasible to discontinue it before doing FMT. In this study, one of the two dogs presenting with severe cobalamin deficiency (below reference interval) at enrolment did not clinically improve after FMT. Very low cobalamin levels have been associated with a poor clinical outcome in dogs with CE30,37, and that might therefore play a role in the lack of a response to FMT in this case.
FMT is increasingly used in veterinary medicine, and to date FMT has resulted in clinical improvement of dogs with CE and puppies with parvovirus infection11,12,15,16,38,39. As a primary treatment, FMT has been described so far in a case report (administered via rectal enema) and a small study with 3 dogs (administered via oral capsules)12,16. At the same time, it has been used as adjunctive therapy in addition to corticosteroids15,38 or a combination of treatments11,38. The present study evaluated the effects of FMT as a primary treatment via retention enema, with a standardised protocol and time of follow-up (90 days after FMT). Recently, a 60-month oral administration has been tested prospectively in 3 dogs with diarrhoea, with 60 days of follow-up afterwards16. To date, the use of FMT via rectal enema has been used in a non-standardised manner, with administrations ranging from 1 to 9 FMT, and time of evaluation ranging from 96 h to 40 months after FMT11,12,38,39. In addition, preparation of inoculum can be different if fresh or frozen faeces are used, and apparently, the effects of FMT might be different based upon that. To date, frozen faeces have been chosen in most studies11,38,39, while here, as done previously by Niina et al.12, fresh faeces were used. However, contrary to our study, in Niina et al.12fresh faeces were collected from multiple donors. From data currently available in the literature, it is not possible to determine if the outcome would be better using fresh versus frozen faeces, or using one versus multiple donors.
In this study, dogs were prospectively evaluated for 90 days after FMT and a clinical improvement was documented in the majority of dogs (17/20). The short-term efficacy of FMT as monotherapy has been previously described in one study involving only 3 dogs with diarrhoea, that aimed to evaluate the effects of oral FMT for 60 days after finishing the protocol, with a good response for 2/3 dogs16. Moreover, the present study confirmed that dogs with a good short-term response to FMT might be free of relapses of gastrointestinal signs for longer, and data showed a lasting good response up to 1 year after FMT for more than one in two dogs (10/17). However, the retrospective nature of the long-term data should be interpreted with caution. In this regard, even longer responses have been recently documented in a study which retrospectively collected data from 41 dogs with CE receiving multiple administrations following the first FMT, but not as monotherapy11. Results from the present study support the use of FMT as an effective therapeutic strategy in non-dietary responders CE dogs, with good clinical response in the short term. Future prospective studies with standardised protocols are required to gather more data regarding the efficacy of FMT in chronic enteropathies in dogs40.
Identifying potentially predictive factors of good clinical outcomes following FMT might help clinicians drive the decision towards using this procedure in clinical settings. The DI is a PCR-based assay developed to quantify intestinal dysbiosis in dogs18. The higher the DI, the more the microbial composition is shifted from normal and the less diverse it is40. In this study, the DI was used to assess microbial shifts over time in response to FMT, as was done in a recent study where it has been shown that dogs with severe dysbiosis are less likely to respond to FMT compared to dogs with less severe DI, regardless of the CIBDAI value11. Results from the current study are in line with Toresson et al11, given that dogs with a long-term good response to FMT had normal DI. To date, studies evaluating the effect of FMT did not report age-related effects in dogs with CE. However, in the present study, dogs with a more favourable response to FMT in terms of normalization of the DI were younger than dogs that did not show an improvement. This result, although based on a limited number of dogs, supports the hypothesis that younger dogs likely present with milder clinical signs and have therefore a better chance of remission with no medical therapy30,37. To summarize based on the findings in this study, with the caution that is required by the small size of the population examined, younger dogs with minor shifts in the microbiota as assessed by DI, with mild clinical signs as assessed by CIBDAI, normal cobalamin levels and faecal calprotectin values within the normal range might respond more positively to FMT compared to dogs with more severe clinical signs and abnormalities on blood and faecal markers.
The present study highlighted that DI improved within one month after FMT in non-diet responsive CE dogs, with significant differences observed among dogs if grouped according to previous antibiotic administration. The results in this study showed that dogs treated with antibiotics between 60 and 30 days before FMT administration had a higher DI compared to dogs free from recent antibiotics administration. Antimicrobial exposure has been strongly associated with perturbation of the intestinal microbiota in humans, dogs and cats41,42. Metronidazole, the most common antimicrobial reported in this study to be previously administered in CE dogs, is a molecule widely used to treat diarrhoea in dogs unresponsive to dietary change43,44. On the other hand, antimicrobials such as metronidazole and tylosin have been associated with long-term induced dysbiosis in dogs40,45,46,47. Therefore, it is hard to differentiate between antimicrobial-induced dysbiosis and CE-associated dysbiosis, which may have had different responses to FMT treatment, in the population of dogs described in this study.
Indeed, dogs in the AB group experienced a positive decrease in DI after FMT, and 80% of them improved from an abnormal DI (> 0) to a normal DI (< 0) after FMT. Before FMT, 57% of dogs from the AB group had a lower abundance of P. hiranonis, a bacterial species important for the conversion of primary to secondary bile acids48,49. P. hiranonis is known to be negatively impacted by metronidazole treatment in dogs40. In the present study, P. hiranonis recovered 30 days after FMT in all but one dog in the AB group, in which it was depleted at first. On the contrary, it has been shown that dogs with persistent low abundance of P. hiranonis both with food-responsive enteropathy29, and steroid-responsive enteropathy50, showed bile acids dysmetabolism with a lower abundance of secondary bile acids. In a study, although dogs fed a homemade diet showed improved clinical signs within 30 days, the dietary treatment was not able to restore P. hiranonis and secondary bile acids abundance29. Another study showed that steroids increased faecal concentration of P. hiranonis and secondary bile acids after 2–3 months of treatment50. Therefore, as per the present study and a previous one45, FMT seems to be a quick and effective method to restore P. hiranonis in dogs with CE, particularly in the case of previous antibiotic treatments. The recovery of P. hiranonis explained the variations in faecal bile acids observed 30 days after FMT in this study, characterized by an overall decrease in primary bile acids and a subsequent increased conversion rate to secondary bile acids.
In the present study the faecal microbiota of dogs with CE refractory to diet was similar to that of clinically healthy dogs based on sequencing. These sequencing results aligned with the quantitative DI assay, where only a minority of the study population (5/20 dogs with CE) had significant dysbiosis at T0. This indicated that these dogs did not have major shifts in the microbiome. While alpha and beta diversity analyses showed no significant differences between dogs with CE and healthy controls, categorizing samples based on DI interpretation revealed that microbiota shifts paralleled the increase in DI. This finding is consistent with previous studies in dogs with various phenotypes, indicating that DI can predict and quantify microbiota shifts to an extent25. These results further suggest that dogs may respond better to FMT when only mild changes in the microbiota and the DI are present.
The genus Erysipelatoclostridium, belonging to Clostridium cluster XVIII, is reported to produce acetate and has been identified with metabolites that have been potentially involved in the regulation of immune homeostasis and chronic inflammation51, thus it is considered a potential marker of intestinal diseases such as Crohn’s disease and Clostridioides difficile infection in humans52. In this study via ANCOM analysis, the genus Erysipelatoclostridium was identified as the only differentially abundant bacterial group, with lower abundance in CE compared to healthy controls, and this result is consistent with a reduced abundance previously reported in dogs with acute diarrhoea53. This specific alteration was not captured by ANCOM-BC, suggesting the need for multiple statistical approaches to better analyse the compositional data. Overall, our findings emphasize that individual-level assessments like DI are crucial for identifying subtle yet significant microbial alterations, offering a method to evaluate faecal microbiota shifts in a clinical setting.
In this study, in line with the fact that the composition of the microbiota was not deeply altered in dogs treated with FMT, no substantial changes in the production of bacterial metabolites (e.g. volatile fatty acids and sterols), or in parameters indirectly related to metabolism such as faecal pH or ammonia were observed. However, a temporary increased faecal propionic acid concentration was measured in this study 30 days after FMT. Like acetic and butyric, propionic acid is a short-chain fatty acid (SCFA) mainly formed during the microbial fermentation of carbohydrates in the colon54. It was found to be lower in faecal samples of dogs with acute and chronic diarrhoea as compared to healthy, and it correlated with the clinical score in CE dogs, making it a potential marker of disease severity dogs55,56,57. Although temporary, the increased level of propionate found in the faeces of CE dogs in this study is, therefore, a positive aspect attributable to FMT that might have played a role in the clinical improvement observed in dogs.Our study had several limitations associated with the nature of clinical trials, including missing samples at follow up and dropouts. Firstly, the diet was not standardised, and we cannot exclude the possibility that this may have had some effect on the results. One additional limitation that needs to be discussed regards the type of CE we inadvertently selected. Because our study was meant to use FMT as a sole therapy, attending clinicians involved in this trial may have introduced a selection bias whereby mostly dogs with mild to moderate clinical disease were considered, as it would be unethical to withhold other potentially life-saving therapies from dogs with severe CE. This was reflected in lower CIBDAI scores and milder shifts in microbiota composition at T0, and better FMT outcomes than could be expected in the general population of dogs with CE. Indeed, a previous study11 has reported that dogs with mild dysbiosis as measured by DI are more likely to respond to FMT, which may explain the positive responses observed in our study.
Conclusions
This study highlights the importance of FMT as an effective therapeutic strategy in dogs presenting with chronic gastrointestinal signs refractory to diet associated with mild intestinal microbial perturbations. In these cases, a treatment targeting microbial modulation, such as FMT, can be applicable after the dietary trial before considering the use of drugs associated with a higher risk of side effects. FMT can promote favourable microbial modulation in dogs previously treated with antimicrobials. The majority of dogs in this study showed a positive clinical response, with evidence of a long-lasting response up to one year. Factors associated with a good outcome following FMT in dogs with chronic enteropathies need to be clarified and confirmed in additional; however, results from this study evidenced that young dogs with signs of mild gastrointestinal disease and milder form of dysbiosis might be good candidates to benefit from treatment with FMT.
Data availability
All data generated and analyzed in this study are included in the published article. The data have been deposited with links to SRA database accession number PRJNA1167336 release date: 2025-01-01. The data presented in this study have been deposited with links to SRA database accession number PRJNA1167336 release date: 2025–01-01.
References.
-
Dandrieux, J. R. S. Inflammatory bowel disease versus chronic enteropathy in dogs: Are they one and the same?. J. Small Anim. Pract. 57, 589–599 (2016).
-
Makielski, K., Cullen, J., O’Connor, A. & Jergens, A. E. Narrative review of therapies for chronic enteropathies in dogs and cats. J. Vet. Intern. Med. 33, 11–22 (2019).
-
Tolbert, M. K., Murphy, M., Gaylord, L. & Witzel-Rollins, A. Dietary management of chronic enteropathy in dogs. J. Small Anim. Pract. 63, 425–434 (2022).
-
Cerquetella, M. et al. Proposal for rational antibacterial use in the diagnosis and treatment of dogs with chronic diarrhoea. J. Small Anim. Pract. 61, 211–215 (2020).
-
Procoli, F. Inflammatory bowel disease, food-responsive, antibiotic-responsive diarrhoea, protein losing enteropathy. Adv. Small Anim. Care 1, 127–141 (2020).
-
Winston, J. A., Small, D. & Internal, A. Clinical guidelines for fecal microbiota transplantation in companion animals. Adv. Small Anim. Care https://doi.org/10.1016/j.yasa.2024.06.006 (2024).
-
Chaitman, J. & Gaschen, F. Fecal microbiota transplantation in dogs. Vet. Clin. N. Am. – Small Anim. Pract. 51, 219–233 (2021).
-
Borody, T. J., Paramsothy, S. & Agrawal, G. Fecal microbiota transplantation: Indications, methods, evidence, and future directions. Curr. Gastroenterol. Rep. 15, 1–7 (2013).
-
Salavati Schmitz, S. Observational study of small animal practitioners’ awareness, clinical practice and experience with fecal microbiota transplantation in dogs. Top Companion Anim. Med. 47, 100630 (2022).
-
Lee, M. A. et al. Safety profile and effects on the peripheral immune response of fecal microbiota transplantation in clinically healthy dogs. J. Vet. Intern. Med. 38, 1425–1436 (2024).
-
Toresson, L. et al. Clinical effects of faecal microbiota transplantation as adjunctive therapy in dogs with chronic enteropathies—a retrospective case series of 41 dogs. Vet. Sci. 10, 1–18 (2023).
-
Niina, A. et al. Fecal microbiota transplantation as a new treatment for canine inflammatory bowel disease. Biosci. Microbiota Food Health 40, 98–104 (2021).
-
Berlanda, M. et al. Case report faecal microbiome transplantation as a solution to chronic enteropathies in dogs: A case study of beneficial microbial evolution. Animals 11, 1433 (2021).
-
Sugita, K. et al. Successful outcome after a single endoscopic fecal microbiota transplantation in a Shiba dog with non-responsive enteropathy during the treatment with chlorambucil. J. Vet. Med. Sci. 83, 984–989 (2021).
-
Cerquetella, M. et al. Case Report: Oral fecal microbiota transplantation in a dog suffering from relapsing chronic diarrhea—clinical outcome and follow-up. Front. Vet. Sci. 9, 1–6 (2022).
-
Carapeto, S. et al. Effect of the administration of a lyophilised faecal capsules on the intestinal microbiome of dogs: A pilot study. Genes (Basel) 14, 1676 (2023).
-
Bottero, E., Benvenuti, E. & Ruggiero, P. Faecal microbiota transplantation in 16 dogs with idiopathic inflammatory bowel disease. Veterinaria 31, 1–12 (2017).
-
AlShawaqfeh, M. K. et al. A dysbiosis index to assess microbial changes in fecal samples of dogs with chronic inflammatory enteropathy. FEMS Microbiol. Ecol. 93, 1–8 (2017).
-
Laflamme, D. P. Development and validation of a body condition score system for dogs. Canine Pract. 22, 10–15 (1997).
-
Jergens, A. E. et al. A scoring index for disease activity in canine inflammatory bowel disease. J. Vet. Intern. Med. 17, 291–297 (2003).
-
Enderle, L. L., Köller, G. & Heilmann, R. M. Verification of the fCAL turbo immunoturbidimetric assay for measurement of the fecal calprotectin concentration in dogs and cats. J. Vet. Diagn. Invest. 34, 813–824 (2022).
-
Pinna, C., Vecchiato, C. G., Delsante, C., Grandi, M. & Biagi, G. On the variability of microbial populations and bacterial metabolites within the canine stool an. in-depth analysis. Animals 11, 225 (2021).
-
Sung, C. H. et al. Fecal concentrations of long-chain fatty acids, sterols, and unconjugated bile acids in cats with chronic enteropathy. Animals 13, 2753 (2023).
-
Galler, A. I. et al. Microbial dysbiosis and fecal metabolomic perturbations in Yorkshire Terriers with chronic enteropathy. Sci. Rep. 12, 1–18 (2022).
-
Sung, C. H. et al. Correlation between targeted qPCR assays and untargeted DNA shotgun metagenomic sequencing for assessing the fecal microbiota in dogs. Animals 13, 2597 (2023).
-
Mandal, S. et al. Analysis of composition of microbiomes: A novel method for studying microbial composition. Microb. Ecol. Health Dis. https://doi.org/10.3402/mehd.v26.27 (2015).
-
Lin, H. & Peddada, S. D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. https://doi.org/10.1038/s41467-020-17041-7 (2020).
-
McMurdie, P. J. & Holmes, S. Phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE https://doi.org/10.1371/journal.pone.0061217 (2013).
-
Vecchiato, C. G. et al. Fecal microbiota, bile acids, sterols, and fatty acids in dogs with chronic enteropathy fed a home-cooked diet supplemented with coconut oil. Animals 13, 502 (2023).
-
Volkmann, M. et al. Chronic diarrhea in dogs – Retrospective study in 136 cases. J. Vet. Intern. Med. 31, 1043–1055 (2017).
-
Holmberg, J. et al. Chronic enteropathy in dogs—Epidemiologic aspects and clinical characteristics of dogs presenting at two swedish animal hospitals. Animals 12, 1507 (2022).
-
Mandigers, P. J. J., Biourge, V., van den Ingh, T. S. G. A. M., Ankringa, N. & German, A. J. A randomized, open-label, positively-controlled field trial of a hydrolyzed protein diet in dogs with chronic small bowel enteropathy. J. Vet. Intern. Med. 24, 1350–1357 (2010).
-
Simpson, K. W. et al. Randomized controlled trial of hydrolyzed fish diets in dogs with chronic enteropathy. J. Vet. Intern. Med. 37, 2334–2343 (2023).
-
Bresciani, F. et al. Effect of an extruded animal protein-free diet on fecal microbiota of dogs with food-responsive enteropathy. J. Vet. Intern. Med. 32, 1903–1910 (2018).
-
Hodel, S., Brugger, D. & Kook, P. H. Long-term evaluation of the initial response to therapy in 60 dogs with chronic inflammatory enteropathy. J. Vet. Intern. Med. https://doi.org/10.1111/jvim.17161 (2024).
-
Kather, S., Grützner, N., Kook, P. H., Dengler, F. & Heilmann, R. M. Review of cobalamin status and disorders of cobalamin metabolism in dogs. J. Vet. Intern. Med. 34, 13–28 (2020).
-
Allenspach, K., Wieland, B., Gröne, A. & Gaschen, F. Chronic enteropathies in dogs: Evaluation of risk factors for negative outcome. J. Vet. Intern. Med. 21, 700–708 (2007).
-
Collier, A. J. et al. Investigating fecal microbial transplant as a novel therapy in dogs with inflammatory bowel disease: A preliminary study. PLoS ONE 17, 1–17 (2022).
-
Pereira, G. Q. et al. Fecal microbiota transplantation in puppies with canine parvovirus infection. J. Vet. Intern. Med. 32, 707–711 (2018).
-
Pilla, R. et al. Effects of metronidazole on the fecal microbiome and metabolome in healthy dogs. J. Vet. Intern. Med. 34, 1853–1866 (2020).
-
Ferrer, M., Méndez-García, C., Rojo, D., Barbas, C. & Moya, A. Antibiotic use and microbiome function. Biochem. Pharmacol. 134, 114–126 (2017).
-
Stavroulaki, E. M., Suchodolski, J. S. & Xenoulis, P. G. Effects of antimicrobials on the gastrointestinal microbiota of dogs and cats. Vet. J. 291, 105929 (2023).
-
Allenspach, K., Culverwell, C. & Chan, D. Long-term outcome in dogs with chronic enteropathies: 203 cases. Vet. Rec. 178, 368 (2016).
-
Langlois, D. K., Koenigshof, A. M. & Mani, R. Metronidazole treatment of acute diarrhea in dogs: A randomized double blinded placebo-controlled clinical trial. J. Vet. Intern. Med. 34, 98–104 (2020).
-
Chaitman, J. et al. Fecal microbial and metabolic profiles in dogs with acute diarrhea receiving either fecal microbiota transplantation or oral metronidazole. Front. Vet. Sci. 7, 1–12 (2020).
-
Manchester, A. C. et al. Long-term impact of tylosin on fecal microbiota and fecal bile acids of healthy dogs. J. Vet. Intern. Med. 33, 2605–2617 (2019).
-
Pinna, C. et al. In vitro evaluation of the effects of tylosin on the composition and metabolism of canine fecal microbiota. Animals 10, 98 (2020).
-
Kitahara, M., Takamine, F., Imamura, T. & Benno, Y. Clostridium a human intestinal bacterium with bile acid 7α-dehydroxylating activity. Int. J. Syst. Evol. Microbiol. 51, 39–44 (2001).
-
Suchodolski, J. S. Diagnosis and interpretation of intestinal dysbiosis in dogs and cats. Vet. J. 215, 30–37 (2016).
-
Guard, B. C. et al. Longitudinal assessment of microbial dysbiosis, fecal unconjugated bile acid concentrations, and disease activity in dogs with steroid-responsive chronic inflammatory enteropathy. J. Vet. Intern. Med. 33, 1295–1305 (2019).
-
Kishikawa, T. et al. A Metagenome-wide association study of gut microbiome in patients with multiple sclerosis revealed novel disease pathology. Front. Cell Infect. Microbiol. https://doi.org/10.3389/fcimb.2020.585973 (2020).
-
Mancabelli, L. et al. Identification of universal gut microbial biomarkers of common human intestinal diseases by meta-analysis. FEMS Microbiol. Ecol. https://doi.org/10.1093/femsec/fix153 (2017).
-
Soonthornsit, J., Ngamwongsatit, N., Sangsuriya, P. & Arya, N. The alterations of fecal microbiota in dogs with acute diarrhea, Thailand. Thai J. Vet. Med. 51, 683–690 (2021).
-
Henningsson, A. M., Björck, I. M. E. & Nyman, E. M. G. L. Combinations of indigestible carbohydrates affect short-chain fatty acid formation in the hindgut of rats. J. Nutr. 132, 3098–3104 (2002).
-
Guard, B. C. et al. Characterization of microbial dysbiosis and metabolomic changes in dogs with acute diarrhea. PLoS ONE 10, 1–24 (2015).
-
Minamoto, Y. et al. Fecal short-chain fatty acid concentrations and dysbiosis in dogs with chronic enteropathy. J. Vet. Intern. Med. 33, 1608–1618 (2019).
-
Higueras, C. et al. Short-chain and total fatty acid profile of faeces or plasma as predictors of food-responsive enteropathy in dogs: A preliminary study. Animals 12, 89 (2022).
Author information
Authors and Affiliations
Contributions
CG.Vecchiato: Investigation, Methodology, Formal analysis, Visualization, Writing—Original Draft, Review & Editing. MC.Sabetti: Investigation, Visualization, Writing -Review, Editing. CH.Sung: Methodology, Formal analysis, Visualization. F.Sportelli: Investigation. C. Delsante: Investigation. C. Pinna: Methodology, Formal analysis. M. Alonzo: Investigation. D. Erba: Investigation. JS. Suchodolski: Methodology, Visualization, Review, Supervision. R.Pilla: Formal analysis, Visualization, Writing—Review, Editing. M. Pietra: Investigation, Visualization, Editing. G.Biagi: Visualization, Supervision. F. Procoli: Conceptualization, Methodology, Writing—Review & Editing, Supervision. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
Drs. Pilla, Suchodolski and Sung are currently employed by the Gastrointestinal Laboratory at Texas A&M University, which offers laboratory tests, including the Dysbiosis Index test, on a fee-for-service basis. Dr Suchodolski is the Purina PetCare Endowed Chair for Microbiome Research and received support for microbiome studies through the Purina PetCare Research Excellence Fund.The other authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vecchiato, C.G., Sabetti, M.C., Sung, C.H. et al. Effect of faecal microbial transplantation on clinical outcome, faecal microbiota and metabolome in dogs with chronic enteropathy refractory to diet.
Sci Rep 15, 11957 (2025). https://doi.org/10.1038/s41598-025-96906-7
-
Received:
-
Accepted:
-
Published:
-
DOI: https://doi.org/10.1038/s41598-025-96906-7
This post was originally published on this site be sure to check out more of their content.