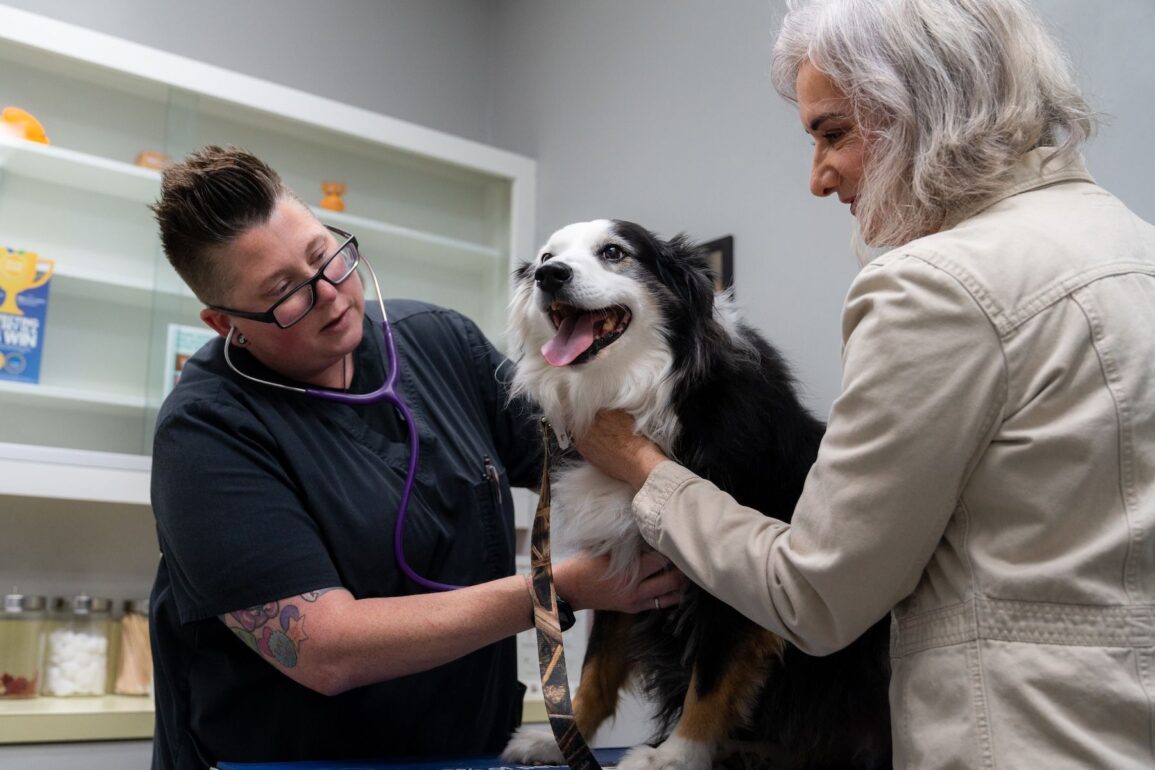
Photo courtesy of Loyal
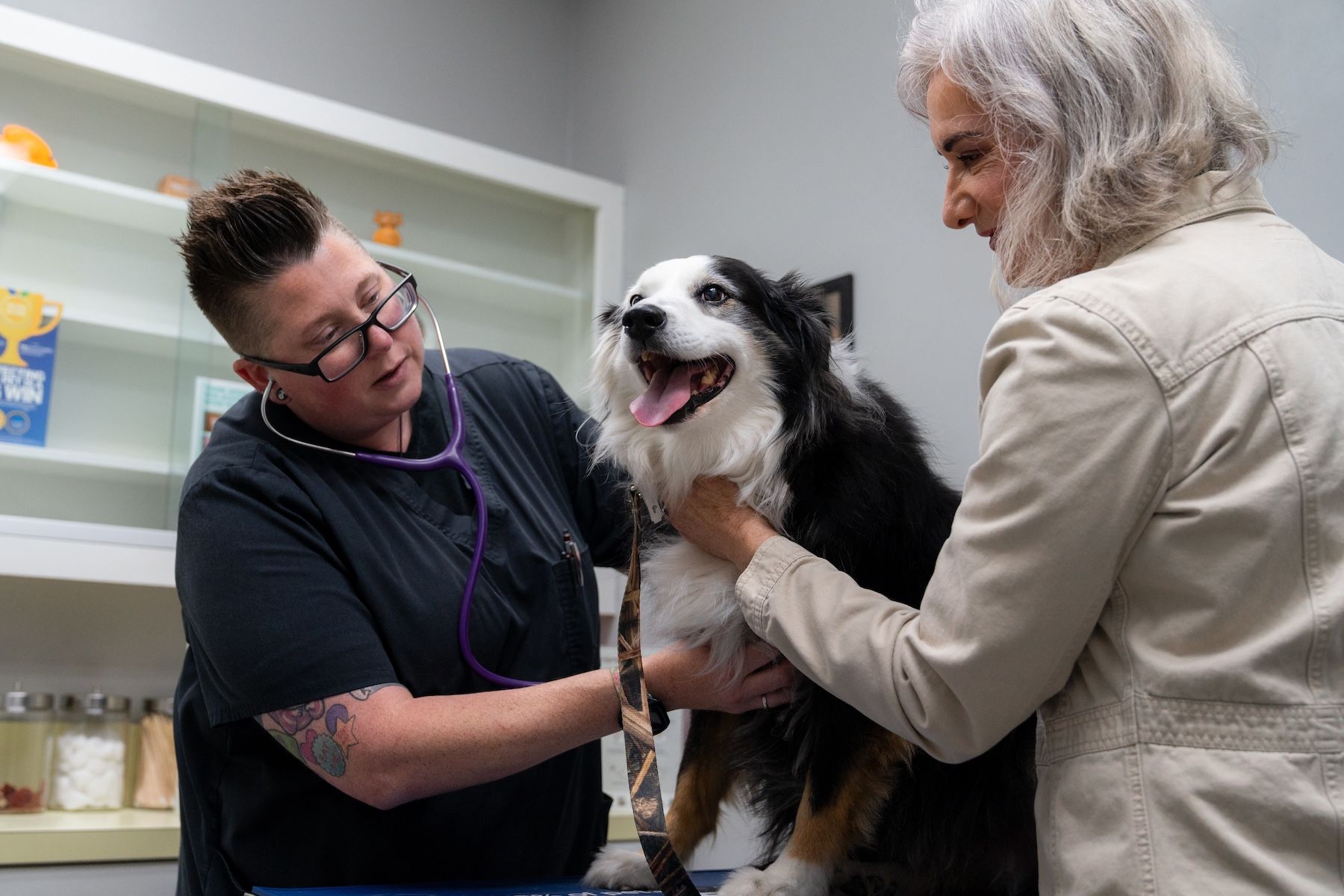
A developmental drug that aims to extend the healthy lifespan of senior dogs has received acceptance by the FDA’s Center for Veterinary Medicine for the Reasonable Expectation of Effectiveness (RXE) section of the conditional approval application submitted by Loyal, a clinical-stage animal health company. The drug, LOY-002, targets age-related metabolic dysfunction and is also designed to improved quality of life in canines aged 10 years and older weighing at least 14 lbs.1
“Loyal’s progress in bringing their products to market continues to impress, especially since they are breaking new regulatory ground,” Linda Rhodes, VMD, PhD, a member of Loyal’s Board of Directors, said in a news release.1
This new RXE is the second to receive acceptance by the FDA for a longevity drug developed by Loyal. The first, LOY-001, received RXE acceptance in November 2023. LOY-001 is a prescription injection that targets large- and giant-breed dogs such as rottweilers, Great Danes and Bernese Mountain dogs, according to Celine Halioua, CEO of Loyal.2
A third longevity drug, LOY-003, is also in Loyal’s pipeline. LOY-003 is a prescription pill that aims to provide the same benefits to the large- and giant-breed canine population as LOY-001. Both LOY-001 and LOY-003 intend to target dogs aged 7 years and older, weighing at least 40 lbs.1
Clinical study
Loyal is currently running the STAY clinical study to further examine LOY-002, and to provide effectiveness and field safety data for its’ FDA application for drug approval. Launched in late 2023, the STAY study is expected to run 4 years with 1000 dogs enrolled at dozens of independent veterinary clinics across the US. Half of the enrolled dogs are receiving a beef-flavored LOY-002 pill while half of the study enrollees receive a placebo.3
In a dvm360 interview, Ellen Ratcliff, DVM, vice president of clinical and veterinary medicine at Loyal, said the study has been running in approximately 70 US veterinary clinics. Participation is being recorded in rural, urban and large metropolitan areas. “[It is] just a lot of really great engagement with veterinarians,” she said. “One of the neat things about the STAY study is that we are using a lot of clinical trial-naïve study sites—veterinarians who have never participated in clinical research or doing clinical research at their clinics.”
The STAY study dosed its’ first dog, a Whippet named Boo, in December 2023. This study is the first and only FDA-concurred clinical trial for longevity and is the largest veterinary trial in history, according to Loyal.1,3
“It doesn’t do anybody any good if their dog lives longer, but they live longer in that period at the end of the life where they don’t feel well, and they’re sick, having all kinds of degenerative and aging diseases. So, the STAY study is also measuring quality of life according to the owner and the owner assessment of the dog’s quality of life,” Ratcliff said.
Financial support
In announcing the RXE for LOY-002, officials with Loyal also said the company has raised $22 million for its’ B-2 round, from investors that include Valor Equity Partners and Collaborative Fund. Combined with $45 million in Series B funding it raised in 2024, Loyal now has investment of more than $150 million.
“Everything we do is in service of helping dogs live longer, healthier lives,” Halioua said in the release. “These 2 milestones represent our ongoing commitment to that mission through years of diligence and hard work. Proving efficacy is one of the most challenging parts of developing a novel drug. While we still have significant work to do, RXE increases the probability that dogs will soon have access to our longevity drugs.”
The funding raised will support Loyal’s efforts to bring LOY-002 to the market for veterinarians and dog owners. These funds will also help advance LOY-001 and LOY-003, according to the company.
Takeaway
Loyal is pursuing the FDA’s expanded conditional approval and company officials anticipate completion of manufacturing and safety requirements for this application by late 2025. Regardless, Ratcliff noted that RXE acceptance is an achievement that serves as a testament to the importance of the STAY study.
“Completing enrollment and seeing what the data shows over the next several years is critical, but this is an important step in the process. We now have validation from one of the highest quality regulatory agencies in the world that the drug we’re testing has a reasonable expectation of extending a dog’s life,” Ratcliff said in the release.1
References
- Loyal receives FDA acceptance of Reasonable Expectation of Effectiveness for senior dog lifespan extension. News release. Loyal. February 26, 2025. Accessed February 26, 2025. https://www.businesswire.com/news/home/20250226676005/en
- Coppock K. FDA determines drug for lifespan extension in large dogs to have a Reasonable Expectation of Effectiveness. dvm360. November 28, 2023. Accessed February 26, 2025. https://www.dvm360.com/view/fda-determines-drug-for-lifespan-extension-in-large-dogs-to-have-a-reasonable-expectation-of-effectiveness
- Coppock K. A clinical trial is launched for a novel drug that could extend healthy lifespan in senior dogs. dvm360. February 1, 2024. Accessed February 26, 2025. https://www.dvm360.com/view/a-clinical-trial-is-launched-for-a-novel-drug-that-could-extend-healthy-lifespan-in-senior-dogs
This post was originally published on this site be sure to check out more of their content.